Acute Cerebral Phaeohyphomycosis due to Wangiella dermatitidis Accompanied by Cerebrospinal Fluid Eosinophilia-Korea
Departments of Clinical Pathology,1 Neurology,2 Neurosurgery,3 Radiology,4 and Anatomic Pathology,5 College of Medicine, Pusan National University, Pusan, and Department of Clinical Pathology, Chonnam University Medical School, Kwangju,6 Korea
Received 20 December 1999/Returned for modification 26 January 2000/Accepted 15 February 2000
|
|
ABSTRACT |
|---|
|
|
|---|
We report a case of cerebral phaeohyphomycosis due to Wangiella dermaitidis in an immunocompetent adult man. His cerebrospinal fluid (CSF) showed pleocytosis with a high eosinophil count but without peripheral blood eosinophilia. The present case suggested that this black yeast-like fungus should be included when the causes of CSF eosinophilia are considered, even though it is an extremely rare pathogen.
|
|
TEXT |
|---|
|
|
|---|
Central nervous system phaeohyphomycosis due to Wangiella dermatitidis (also known as Exophiala dermatitidis) is extremely rare, and documented cases have not occurred outside of Asia. So far, only six documented cases of W. dermatitidis as a causative organism of brain infections have been reported in the world. There have also been three more probable cases of brain infections, in which W. dermatitidis was isolated from body tissues other than the brain (1, 5, 8, 9). However, cases of cerebral phaeohyphomycosis accompanied by cerebrospinal fluid (CSF) pleocytosis and eosinophilia have not been reported as yet, and black yeasts, including W. dermatitidis, are not included in the long list of probable causes of CSF eosinophilia (12). We report a case of cerebral phaeohyphomycosis caused by W. dermatitidis with a high CSF eosinophil count.
Case
report. A 28-year-old male was admitted to the neurology ward of Pusan
National University Hospital because of a headache that worsened
over 5 days. Five days before his admission to the hospital, he
had first noticed a diffuse, pulsating headache upon awakening in
the morning. The following day, his headache worsened and was
accompanied by nausea and vomiting. One day before his admission
to the hospital, a severe headache woke him up at night. He was
otherwise a healthy engineer working for a multinational company
and had traveled for 3 years to many countries in Southeast and
Middle East Asia, North America, South America, and Europe. He
had no history of any other systemic diseases, such as tuberculosis,
allergies, diabetes mellitus, or hypertension. He denied any history
of smoking, habitual drinking, or drug abuse. He had normal blood
pressure (120/60 mm Hg), pulse rate (65/min), and respiratory
rate (19/min), and he was afebrile (36.5°C). The results of his
general physical examination were nonspecific. A detailed neurological
examination also did not reveal any abnormalities, such as neck
stiffness, hemiparesis, pupillary change, or pathologic reflexes.
A computerized tomography view of the brain showed multiple
ill-defined low-density lesions, and a magnetic resonance view
showed multiple small enhancing lesions. A spinal tap revealed
high opening pressure (290 mm H2O), and a CSF analysis
showed pleocytosis (290/mm3, 60% lymphocytes), an increased level
of protein (128 mg/ml), and a normal sugar level (96 mg/ml). A
CSF Gram and acid-fast bacillus staining and India ink
preparation yielded negative results. A high proportion of
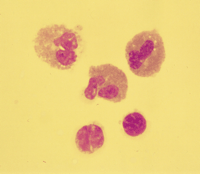
eosinophils was noticed on CSF cytology (40%) (Fig.
1). The
results of the enzyme-linked immunosorbent assay for Paragonimus
and Cysticercus were negative. An intravenous osmotic agent,
steroid, and oral praziquantel were started 2 days after the
patient's admission, producing significant symptomatic
improvement. On day 6 of hospitalization, the patient had a constant,
severe headache with nausea and projectile vomiting, which did
not respond to intravenous analgesics or osmotic agents. A control
brain magnetic resonance view on day 8 revealed obstructive
hydrocephalus. On day 10, surgery was performed to relieve the
obstructive hydrocephalus. Upon operation, it was revealed that
the lateral ventricle was filled with dirty, cloudy CSF, with
severe adhesion around the entry of the third ventricle. A biopsy
of the ventricular wall was performed, and an extraventricular
drainage catheter was placed in the lateral ventricle. A frozen
biopsy specimen showed scattered foci of tangled fungi with
mold-like features in addition to an intense inflammatory
reaction. A fungal culture was performed, and 50 mg of
amphotericin B was administered daily. After the operation, the
patient was alert and oriented but his headache persisted, with
nausea and vomiting. On day 12, the patient suddenly lost
consciousness, and after that, he showed a stuporous to comatose
mental status. He expired on day 13.
|
Mycology. Three days after incubation at room temperature and 37°C on Sabouraud's dextrose agar, the colonies appeared black, reverse black, wet, and mucoid and could be picked up as a string of material from the plate to an inoculating loop. Sparse, septate, and pale olivaceous hyphae were observed under a microscope. Black yeast synanamorphs were abundant. Conidiogenous cells were cylindrical, with rounded apices producing one-celled conidia. Round to ovoid, pale brown conidia accumulated in balls or slipped down the side of conidiophore. The organism was identified as W. dermatitidis because of its ability to grow at 40°C and its lack of nitrate assimilation.
W. dermatitidis infections that involve the brain appear to occur only or predominantly in Asian people. All seven documented cases, including the present case, have been from Asian countries: Japan (three), Taiwan (two), Pakistan (one), and Korea (one) (1, 5, 8, 9). There have also been probable cases from Japan (two) and the United States (one), in which lesions caused by W. dermatitidis were found in multiple body sites but the exact causes of the lesions in the brain were not confirmed (8, 9). Even in immunocompetent hosts the brains were infected, and the outcomes were invariably fatal.
On the other hand, the occurrence of W. dermatitidis infections in other body sites is not restricted to specific areas of the world. To date, there have been 39 documented cases of W. dermatitidis infection that do not involve the brain (1-10, 13, 14). The patients were usually immunocompromised patients. Sixteen cases were from Japan, 11 were from the United States, 2 each were from France, The Netherlands, and the United Kingdom, and 1 each was from Brazil, Czechoslovakia, Germany, Korea, Singapore, and Spain. Therefore, it has been widely seen that W. dermatitidis is one of the etiologic agents of phaeohyphomycosis in various sites of the body, including the subcutaneous tissue, and of fungemia, especially in immunocompromised hosts. It is noteworthy, however, that neurotropism of this fungus has almost always been restricted to Asian countries and that the immunocompetent host was usually suffering from cerebral phaeohyphomycosis. This suggests that genetic factors may contribute to the progression of this organism in the brain.
One more interesting point in the present case is CSF eosinophilia. CSF pleocytosis was not described in any of the nine previously reported cases of cerebral W. dermatitidis infections, including the three probable cases. In another supposed cerebral W. dermatitidis infection, CSF pleocytosis (250 to 1,300/mm3) with 30 to 70% eosinophils was shown (11). However, in the review of Matsumoto et al., the causative organism was not available for a reconfirmation of its identification, and the reviewers were not sure of the cause of the infection (9). Even if the case that was reported by Nakano et al. (11) was caused by W. dermatitidis, it is extremely rare for CSF pleocytosis and eosinophilia to accompany cerebral phaeohyphomycosis due to W. dermatitidis. To date, the list of main causes of CSF eosinophilia includes inflammatory or infectious diseases, such as cerebral cysticercosis, viral encephalitis, and myelitis, and does not include fungal infection (12). The present case suggests that, in cases of multiple brain abscesses accompanied by CSF eosinophilia, W. dermatitidis infection should be included in a differential diagnosis.
|
|
FOOTNOTES |
|---|
* Corresponding author. Mailing address: Department of Clinical Pathology, College of Medicine, Pusan National University, #10 1-Ga Ami-Dong Seo-Gu, Pusan 602-739, Korea. Phone: 82-51-240-7418. Fax: 82-51-247-6560. E-mail: cchl@hyowon.cc.pusan.ac.kr.
|
|
REFERENCES |
|---|
|
|
|---|
| 1. | Ajanee, N., M. Alam, K. Holmberg, and J. Khan. 1996. Brain abscess caused by Wangiella dermatitidis: case report. Clin. Infect. Dis. 23:197-198[Medline]. |
| 2. | Benaoudia, F., M. Assouline, Y. Pouliquen, A. Bouvet, and E. Gueho. 1999. Exophiala (Wangiella) dermatitidis keratitis after keratoplasty. Med. Mycol. 37:53-56[CrossRef][Medline]. |
| 3. | Blaschke-Hellmessen, R., I. Lauterbach, K. D. Paul, K. Tintelnot, and G. Weissbach. 1994. Detection of Exophiala dermatitidis (Kano) De Hoog 1977 in septicemia of a child with acute lymphatic leukemia and in patients with cystic fibrosis. Mycoses 37(Suppl. 1):89-96[Medline]. |
| 4. | Gerard, C., B. Duchesne, M. P. Hayette, B. Lavalleye, and C. Marechal-Courtois. 1998. A case of Exophiala dermatitidis ulceration. Bull. Soc. Belge Ophtalmol. 268:103-108[Medline]. |
| 5. | Hiruma, M., A. Kawada, H. Ohata, Y. Ohnishi, H. Takahashi, M. Yamazaki, A. Ishibashi, K. Hatsuse, M. Kakihara, and M. Yoshida. 1993. Systemic phaeohyphomycosis caused by Exophiala dermatitidis. Mycoses 36:1-7[Medline]. |
| 6. | Kabel, P. J., K. E. Illy, R. A. Holl, A. G. Buiting, and R. G. Wintermans. 1994. Nosocomial intravascular infection with Exophiala dermatitidis. Lancet 344:1167-1168[Medline]. |
| 7. | Lye, W. C. 1993. Peritonitis due to Wangiella dermatitidis in a patient on CAPD. Peritoneal Dial. Int. 13:319-320. |
| 8. | Matsumoto, T., T. Matsuda, M. R. McGinnis, and L. Ajello. 1993. Clinical and mycological spectra of Wangiella dermatitidis infections. Mycoses 36:145-155[Medline]. |
| 9. | Matsumoto, T., A. A. Padhye, L. Ajello, and P. G. Standard. 1984. Critical review of human isolates of Wangiella dermatitidis. Mycologia 76:232-249. |
| 10. | Nachman, S., O. Alpan, R. Malowitz, and E. D. Spitzer. 1996. Catheter-associated fungemia due to Wangiella (Exophiala) dermatitidis. J. Clin. Microbiol. 34:1011-1013[Abstract]. |
| 11. | Nakano, S., and K. Asano. 1967. Two cases of chromoblastomycosis. Igakunoayumi 61:216-218. |
| 12. | Sa, M. J., C. A. Silva, and C. Cruz. 1986. Clinical and CSF cyto-proteic findings in 23 patients with CSF eosinophilia. Acta Neurol. Scand. 73:279-282[Medline]. |
| 13. | Simpson, A. J., and J. M. Nightingale. 1995. Intravascular line infection with Exophiala dermatitidis. Lancet 345:67[Medline]. |
| 14. | Woollons, A., C. R. Darley, S. Pandian, P. Arnstein, J. Blackee, and J. Paul. 1996. Phaeohyphomycosis caused by Exophiala dermatitidis following intra-articular steroid injection. Br. J. Dermatol. 135:475-477[Medline]. |
Journal of
Clinical Microbiology, May 2000, p. 1965-1966, Vol. 38, No. 5
0095-1137/00/$04.00+0
Copyright © 2000,
American Society for
Microbiology. All rights reserved.