Mechanisms of protection against aflatoxin B1 genotoxicity in rats treated by organosulfur compounds from garlic
Institut National de la Recherche Agronomique, Unité Mixte de Recherche de Toxicologie Alimentaire, BP 86510, 17 rue Sully, 21065 Dijon Cedex, France
|
|
Abstract |
|---|
|
|
|---|
Diallyl sulfide (DAS) and diallyl disulfide (DADS), two garlic
constituents, were found previously to inhibit aflatoxin B1
(AFB1)-initiated carcinogenesis in rat liver, DADS being
the most effective. In order to study the mechanisms involved in
this protection, we have examined the ability of liver microsomes
and cytosols from DAS- and DADS-treated rats to modulate the
mutagenicity and the metabolism of AFB1. We also examined
the effects of these compounds on the expression of cytochromes
P450 (CYP) and phase II enzymes known to be involved in AFB1
metabolism. Administration of DAS (1 mmol/kg for 4 days) to
rats resulted in significant inhibition of microsome-mediated
mutagenicity of AFB1, whereas DADS treatment did not alter AFB1
mutagenicity. DAS treatment increased the metabolism of AFB1
mainly towards the formation of AFQ1 and AFM1,
which might account for the reduction of AFB1
microsomal-mediated mutagenicity. DADS treatment slightly
affected the oxidative metabolism of AFB1. DAS and
DADS induced CYP3A2, CYP2B1 and CYP2B2, DAS being more potent.
Cytosols from DAS- and DADS-treated rats produced a significant
inhibition of AFB1-8,9-epoxide (AFBO)-induced
mutagenicity and significantly increased the cytosolic formation
of AFB1-glutathione conjugates, DADS treatment being more
effective. Western blot analysis showed that DADS is a potent
inducer of glutathione S-transferase A5 (rGSTA5) and AFB1
aldehyde reductase 1 (rAFAR1), while DAS is a weak inducer of
these enzymes. Finally, we demonstrated that antibodies raised
against rGSTA5 strongly reduced the antimutagenic activity of
cytosols from DAS- and DADS-treated rats against AFBO. All
together, these results demonstrate that DAS prevents AFB1
mutagenicity through a dual mechanism, i.e. by modulating both
the phase I and II metabolism of AFB1, whereas DADS
acts mainly by increasing the phase II metabolism of AFB1.
The induction of rGSTA5 and rAFAR1 is probably the main mechanism
by which allyl sulfides give protection against AFB1-induced
carcinogenesis.
Abbreviations: AFAR, aflatoxin aldehyde reductase; AFB1, aflatoxin B1; AFBO, AFB1-8,9-epoxide; BHT, butylhydroxytoluene; CYP, cytochromes P450; DAS, diallyl sulfide; DADS, diallyl disulfide; EQ, ethoxyquin; GSH, glutathione; GST, glutathione S-transferases; I3C, indole-3-carbinol; NQO, NAD(P)H:quinone oxidoreductase
|
|
Introduction |
|---|
|
|
|---|
The mycotoxin aflatoxin B1 (AFB1) is commonly found in
the diet of people in certain areas of the world as a contaminant
in foodstuffs such as corn, peanuts and cotton seeds. This
mycotoxin has been proved to be a very potent hepatocarcinogen in
many species, including rats and primates (1).
Furthermore, epidemiologic studies have demonstrated that
exposure to aflatoxins through the diet, in conjunction with
chronic infection with hepatitis B virus, is one of the major
etiologic factors causing human hepatocellular carcinoma in
southeast China and southern Africa (2,3).
AFB1 requires metabolic activation to exert its carcinogenic action. Cytochromes P450 (CYP) are primarily responsible for activation of AFB1 to the ultimate carcinogen AFB1-8,9-epoxide (AFBO) (1). The exo form of this highly reactive electrophile can readily form adducts with DNA (4). In rats, CYP2C11 and CYP3A2 have been reported to catalyze this activation step (5,6). AFB1 CYP-mediated oxidation can also yield several hydroxylated metabolites, AFM1, AFP1 and AFQ1 (1). Investigations have indicated that CYP1A, CYP2B and CYP3A are involved in the formation of these metabolites which are considered as detoxification products (1,6,7). Another major detoxification pathway of AFB1 in mammalian species is the glutathione (GSH) conjugation of AFBO, which is catalyzed by glutathione S-transferases (GST) (8). Experimental studies conducted in rats have shown that rGSTA5, barely expressed in adult male liver, exhibits a greater activity (at least 100-fold) towards AFBO than other GST subunits (9–11). Interestingly, chemopreventive agents such as ethoxyquin (EQ), oltipraz, butylhydroxytoluene (BHT), coumarin or indole-3-carbinol (I3C) are efficient inducers of rGSTA5 in rats (7,11–13). The induction of rGSTA5, by enhancing the detoxification of AFBO, appears to be one major mechanism that contributes to the protective effect of these chemicals against AFB1-induced pre-neoplastic lesions in the rat (9,11). Moreover, resistance of mice to the deleterious effect of AFB1 is related to a high constitutive expression of mGSTA3, an ortholog form of rat GSTA5, in the liver (14). Thus, overexpression of the GSTA5 subunit plays a major role in protection against AFB1 toxicity. In addition to GST-mediated conjugation of AFB1 with GSH, it has been proposed that AFB1 aldehyde reductase (AFAR) can also reduce the cytotoxicity of AFB1 by preventing the binding of the dialdehydic form of the mycotoxin to intracellular proteins (15). Recent studies have shown that a number of chemopreventive agents, including EQ, induce AFAR in rats (7,12,16).
Numerous epidemiologic and experimental studies imply that garlic can be considered as a dietary anticancer component. Epidemiologic studies have reported that high consumption of garlic reduces the risk of gastric and colon cancer (17). Experimental investigations have provided evidence that organosulfur compounds, present in high amounts in garlic, account for its anticarcinogenic activity (18). Some of these, namely diallyl sulfide (DAS) and diallyl disulfide (DADS), have been shown to inhibit chemically induced carcinogenesis. The protection offered by these organosulfur compounds can occur in several tissues and is effective against a broad-range of carcinogens (19–24). DAS and DADS demonstrated strong anticarcinogenic effects against AFB1-induced hepatocarcinogenesis when they were administered to rats during the initiation phase (25). The mechanisms responsible for these chemopreventive effects have not been fully elucidated. One hypothesis is that DAS and DADS act as blocking agents by enhancing the detoxification pathways of AFB1 as they are able to modify liver CYP and phase II enzymes involved in AFB1 metabolism. DAS and DADS are inducers of CYP1A and CYP2B families and efficient inhibitors of CYP2E1 in rat liver (26–29). In addition, both compounds strongly induce detoxification enzymes such as GST, epoxide hydrolase (EH), NAD(P)H:quinone oxidoreductase (NQO) and UDP-glucuronosyltransferase (UGT) in rat liver, with DADS being the most effective (26,27,30–32). DADS and DAS induce the major hepatic GST subunits and especially GST belonging to the alpha and mu classes (30,32). Moreover, liver GSTP1 has been shown to be highly inducible by DADS but not by DAS (33). The effects of sulfur compounds on the expression of the GSTA5 subunit, which is involved in AFB1 detoxification, have been little explored.
In order to study the mechanisms involved in the inhibition of AFB1 carcinogenesis by DAS and DADS, we have examined here the ability of liver subcellular fractions from DAS- and DADS-treated rats to modulate the activation and the detoxification of AFB1. In addition, the effects of both compounds on the expression of different CYP involved in the metabolism of AFB1 and on the expression of rGSTA5 and rAFAR1 were also assessed. The effects of DAS and DADS were compared with those of EQ, a known inducer of rGSTA5 and rAFAR1 in rat liver (9,16).
|
|
Material and methods |
|---|
|
|
|---|
Chemicals
DAS (purity 97%), DADS (purity 80%, remainder other allyl sulfides),
AFB1 and EQ were obtained from Sigma-Aldrich Chimie
(Saint-Quentin Fallavier, France) and were used without further
purification. Analysis of DADS performed in our laboratory showed
that other allyl sulfides were diallyl trisulfide (18%) and DAS
(2%). [14C]AFB1 (specific activity 80 mCi/mmol)
and AFBO (a 90:10 mixture of the exo and endo
forms, respectively) were obtained from Moravek Biochemicals
(Brea, CA). [14C]AFB1 was diluted with non-radioactive
AFB1 in DMSO to obtain the required specific radioactivity.
Polyclonal antibodies against CYP2C11 were obtained from Gentest
(Woburn, MA). Polyclonal antibodies against CYP2B1/2 were a
generous gift from Prof. A.-M.Batt (Centre du Médicament, Nancy,
France). Polyclonal antibodies against CYP3A1/2 were kindly
provided by Prof. P.Beaune (INSERM U490, Paris, France).
Polyclonal antibodies against rGSTA5 and rAFAR1 were kindly
donated by Prof. J.D.Hayes (Dundee University, Dundee, UK).
Salmonella typhimurium strain TA100 was provided by Dr B.Ames
(Department of Biochemistry, University of California, Berkeley,
CA). Other chemicals were of the highest quality available.
Animals and
treatments
Thirty-two male SPF Wistar rats, 5 weeks old, from Iffa Credo (L’Arbresle,
Lyon, France), were housed in individual stainless steel cages
and maintained at 21°C, with constant humidity and a 12 h
light–dark cycle. They were allotted to four groups of eight.
During the experiment, rats were allowed free access to a
semi-liquid purified diet as described previously (28).
After 2 weeks of feeding, DAS and DADS (1 mmol/kg) were
administered by gavage for 4 consecutive days as described previously
(34).
EQ (0.5%, w/w) was incorporated in the diet for 6 consecutive
days before death. Control rats received vehicle only (corn oil).
Preparation of
hepatic subcellular fractions
Twenty-four hours after the last treatment, the animals were
killed by cervical dislocation following 16 h of fasting. Livers
were removed and pooled. Liver microsomes and cytosols were
prepared as described previously (28,34)
and were stored in aliquots at –80°C. Protein levels were
measured by the method of Bradford (35),
adapted for automatic measurement using a Cobas Fara II
centrifugal analyzer (Roche Instruments, Basel, Switzerland).
CYP3A1 enzyme
activity assay
Nifedipine oxidase (NO) activity, a marker of CYP3A1, was measured
by a HPLC method as described previously (36).
In vitro metabolism
of AFB1
Microsome-mediated metabolism of AFB1 was carried out as
described previously (37).
Briefly, hepatic microsomes (1.5 mg/ml) were incubated for 30 min
at 37°C with 10 µM of [14C]AFB1 (0.05 µCi)
in 80 mM Tris–60 mM KCl buffer pH 7.4, containing 2 mM NADPH and
6 mM MgCl2. After terminating the reaction with cold
methanol, proteins were sedimented by centrifugation and an
aliquot of the supernatant was analyzed by HPLC. Radioactivity
associated with proteins and supernatant was measured in a Packard
scintillation counter.
Cytosol-mediated conjugation of AFB1 to GSH was measured by the quantification of AFB1–GSH conjugates by HPLC. The activation of AFB1 was achieved using chicken liver microsomes, which have the capacity to generate high amounts of AFBO (38). In a total volume of 250 µl, chicken microsomes (1.5 mg/ml) and rat cytosolic fractions (3 mg/ml) were incubated for 30 min at 37°C with 10 µM of [14C]AFB1 (0.05 µCi) in 0.1 M phosphate buffer (pH 7.4) containing NADPH (2 mM), MgCl2 (6 mM) and GSH (5 mM). The reaction was terminated by the addition of 100 µl of cold methanol. The mixture was stored at –20°C for 2 h after which the proteins were precipitated by centrifugation (10 min, 14 000 r.p.m.). An aliquot of the supernatant (50 µl) was injected into a NH2 Uptisphere column (5 µm, 150 x 4.6 mm; Interchim, Montlucion, France) at 40°C, coupled to a radioactivity detector (Radiomatic, Packard, Rungis, France). [14C]AFB1–GSH conjugates were isocratically eluted using the method of Tsikas and Brunner (39). [14C]AFB1–GSH conjugates were identified by testing the absolute dependence on the presence of cytosolic proteins in the incubation medium and by comparison with HPLC retention time of AFB1–GSH conjugates generated by incubation of AFBO with [3H]GSH (Amersham Pharmacia Biotech, Orsay, France).
Western blot
immunoassays
Immunoblot procedures were performed as described previously (28).
For the detection of rGSTA5, hepatic cytosols from male mice were
used as a positive control as antibodies raised against rGSTA5
cross-react with the ortholog murine GSTA3 which has the same
electrophoretic properties as rGSTA5 (9).
Rat kidney cytosols were used as a positive control for the
detection of rAFAR1 because substantial amount of this protein
was found in kidney (40).
The immunoblot quantification of CYP2C11, CYP3A2 and AFAR1 was
carried out using an image analyzer (Bioscan Optimetric, Edmonds,
WA).
Mutagenicity assays
The Ames test was performed with S.typhimurium TA100 according
to Maron and Ames (41)
with slight modifications (33,34).
The effects of hepatic subcellular fractions on the mutagenicity
of AFB1 and AFBO were determined by a liquid pre-incubation
method. AFB1 was pre-incubated at 37°C for 60 min with the
bacteria, microsomes (10%, v/v) and a NADPH-generating system
supplemented with glucose-6-phosphate dehydrogenase (1 U/plate).
The modulation of the mutagenicity of AFBO was carried out by
incubating the mutagen with cytosols (2.5%, v/v) in the presence
of GSH (5 mM). Owing to the great instability of AFBO in aqueous
solution, AFBO was added at the last moment to the incubation
mixture and the duration of the pre-incubation was shortened
to 5 min. This duration is sufficient to allow efficient metabolism
of AFBO by cytosolic enzymes (data not shown). After the
pre-incubation period, the mixtures were diluted with soft agar
and plated onto minimal glucose agar plates. The number of His+
revertants was counted after 48 h incubation at 37°C on two
repetitions of triplicate plates for each dose of mutagen.
Mutagenicity
immunoinhibition studies
The involvement of rGSTA5 in the inhibition of the mutagenicity
of AFBO produced by cytosols was studied using antibodies raised
against rGSTA5. The mutagenicity test was done as described above
except that cytosols were pre-incubated with antibodies raised
against rGSTA5 for 20 min at 37°C before performing the
mutagenicity test. Antibodies were used at a dilution of 1:200.
Statistical
analysis
Data were submitted to an analysis of variance followed by Dunnett’s
test, at P ![]() 0.05, to
compare the treated groups with the control group. Calculations
were done using the SAS System (Cary, NC).
0.05, to
compare the treated groups with the control group. Calculations
were done using the SAS System (Cary, NC).
|
|
Results |
|---|
|
|
|---|
Effects of microsomal fractions on the mutagenicity and metabolism
of AFB1, and on CYP expression
In order to assess the effects of organosulfur compounds on the
activation of AFB1, we measured the modulation of AFB1
mutagenicity by hepatic microsomes from DAS- and DADS-treated
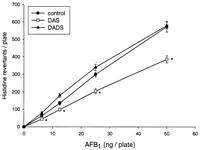
rats in the Ames test (Figure 1![]() ).
DAS significantly reduced the activation of AFB1 when
compared with the control. DADS did not modify AFB1-induced
mutagenicity. To explore the underlying mechanisms, we
investigated the effects of DAS and DADS on the microsome-mediated
metabolism of AFB1 and on the expression of CYPs.
The microsome-mediated metabolism of AFB1 was modified
by DAS treatment and, to a lower extent, by DADS treatment. DAS
significantly increased the formation of Tris-diol, AFM1
and AFQ1 by 1.3-, 1.5- and 1.5-fold, respectively
(Table I
).
DAS significantly reduced the activation of AFB1 when
compared with the control. DADS did not modify AFB1-induced
mutagenicity. To explore the underlying mechanisms, we
investigated the effects of DAS and DADS on the microsome-mediated
metabolism of AFB1 and on the expression of CYPs.
The microsome-mediated metabolism of AFB1 was modified
by DAS treatment and, to a lower extent, by DADS treatment. DAS
significantly increased the formation of Tris-diol, AFM1
and AFQ1 by 1.3-, 1.5- and 1.5-fold, respectively
(Table I![]() ).
DADS treatment only enhanced the formation of AFM1.
Western blot analysis showed that the protein level expression of
CYP2C11, a major CYP in the liver of adult male rat, was not
modified by DAS (data not shown). DADS induced a slight decrease
of CYP2C11 expression but this effect was not significant.
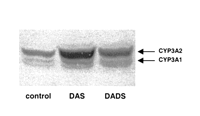
Immunoblotting analysis with antibodies raised against CYP3A1/2
showed that DAS and DADS significantly increased the level of
CYP3A2 (Figure 2
).
DADS treatment only enhanced the formation of AFM1.
Western blot analysis showed that the protein level expression of
CYP2C11, a major CYP in the liver of adult male rat, was not
modified by DAS (data not shown). DADS induced a slight decrease
of CYP2C11 expression but this effect was not significant.
Immunoblotting analysis with antibodies raised against CYP3A1/2
showed that DAS and DADS significantly increased the level of
CYP3A2 (Figure 2![]() ).
The increase in CYP3A2 protein level produced by DAS was higher
(5.1-fold) than that produced by DADS (2.7-fold). DAS and DADS
modified neither CYP3A1 protein expression (Figure 2
).
The increase in CYP3A2 protein level produced by DAS was higher
(5.1-fold) than that produced by DADS (2.7-fold). DAS and DADS
modified neither CYP3A1 protein expression (Figure 2![]() )
nor nifedipine oxidase activity, an enzymatic marker of CYP3A1
(Table II
)
nor nifedipine oxidase activity, an enzymatic marker of CYP3A1
(Table II![]() ).
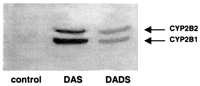
Both DAS and DADS increased the levels of CYP2B1 and CYP2B2, the
proteins, which were not detectable in control (Figure 3
).
Both DAS and DADS increased the levels of CYP2B1 and CYP2B2, the
proteins, which were not detectable in control (Figure 3![]() ).
DAS was the most effective inducer of CYP2B, with a remarkable
induction of CYP2B1. These effects on CYP2B expression are
consistent with the induction of pentoxyresorufin O-alkylase
activity that we observed previously (34).
In contrast, DAS and DADS were ineffective in inducing the
protein levels of CYP1A1 and CYP1A2 (data not shown).
).
DAS was the most effective inducer of CYP2B, with a remarkable
induction of CYP2B1. These effects on CYP2B expression are
consistent with the induction of pentoxyresorufin O-alkylase
activity that we observed previously (34).
In contrast, DAS and DADS were ineffective in inducing the
protein levels of CYP1A1 and CYP1A2 (data not shown).
|
|
|
|
|
Effects of cytosolic fractions on the mutagenicity and metabolism of
AFB1 and on GSTA5 and AFAR1 expression
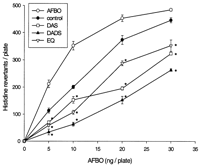
The effects of hepatic cytosols from DAS-, DADS- and EQ-treated
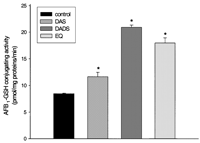
rats on the mutagenicity of AFBO are shown in Figure 4![]() .
All treatments significantly inhibited AFBO-induced mutagenicity
as compared with the control. DADS showed the strongest antimutagenic
activity. Cytosols from DAS- and EQ-treated rats reduced
AFBO-mutagenicity to a similar extent.
.
All treatments significantly inhibited AFBO-induced mutagenicity
as compared with the control. DADS showed the strongest antimutagenic
activity. Cytosols from DAS- and EQ-treated rats reduced
AFBO-mutagenicity to a similar extent.
|
The ability of cytosols from DAS-, DADS- and EQ-treated rats to
conjugate AFB1 to GSH is presented in Figure 5![]() .
DADS-, DAS- and EQ-treatments significantly increased the
formation of AFB1–GSH conjugates when compared with
the control. Cytosols from DADS- and EQ-treated rats were more
efficient in conjugating AFB1 to GSH (2.5- and
2.1-fold, respectively) than those from DAS-treated rats
(1.4-fold).
.
DADS-, DAS- and EQ-treatments significantly increased the
formation of AFB1–GSH conjugates when compared with
the control. Cytosols from DADS- and EQ-treated rats were more
efficient in conjugating AFB1 to GSH (2.5- and
2.1-fold, respectively) than those from DAS-treated rats
(1.4-fold).
|
Immunoblot analysis of rGSTA5 was carried out to determine whether
the antimutagenic activity of DAS, DADS and EQ against AFBO
and their capacity to metabolize AFB1 was related to the increase
of this GST subunit (Figure 6![]() ).
The protein level of rGSTA5 was strongly induced by DADS and EQ
treatments in a similar way whereas rGSTA5 was not detectable in
the control. DAS also induced rGSTA5 although to a lesser extent
than DADS and EQ.
).
The protein level of rGSTA5 was strongly induced by DADS and EQ
treatments in a similar way whereas rGSTA5 was not detectable in
the control. DAS also induced rGSTA5 although to a lesser extent
than DADS and EQ.
|
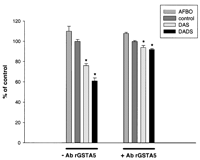
In order to confirm the involvement of the rGSTA5 subunit in the
antimutagenic activity of DAS and DADS against AFBO, we incubated
the hepatic cytosols with polyclonal antibodies raised against
rGSTA5 before doing the Ames test. The antimutagenic activity of
both DAS and DADS treatments was markedly reduced when cytosols
were incubated with rGSTA5 antibodies (Figure 7![]() ).
Without pre-incubation with antibodies, cytosols from DADS- and
DAS-treated rats inhibited AFBO mutagenicity by 40 and 25%,
respectively, when compared with the control, whereas after the
pre-incubation step, inhibition was only 8%. The incubation of
cytosols from control rats with antibodies against rGSTA5 did not
modify the effect of these cytosols against AFBO mutagenicity.
).
Without pre-incubation with antibodies, cytosols from DADS- and
DAS-treated rats inhibited AFBO mutagenicity by 40 and 25%,
respectively, when compared with the control, whereas after the
pre-incubation step, inhibition was only 8%. The incubation of
cytosols from control rats with antibodies against rGSTA5 did not
modify the effect of these cytosols against AFBO mutagenicity.
|
Western blot analysis showed that large amounts of rAFAR1 were
observed in hepatic cytosols from animals treated with EQ and
DADS (Figure 8![]() ).
The increase in rAFAR1 protein level produced by DADS (5.5-fold)
was similar to that produced by EQ (6-fold). DAS-treatment was
also found to increase the level of AFAR protein by 2.5-fold.
).
The increase in rAFAR1 protein level produced by DADS (5.5-fold)
was similar to that produced by EQ (6-fold). DAS-treatment was
also found to increase the level of AFAR protein by 2.5-fold.
|
|
|
Discussion |
|---|
|
|
|---|
One of the major factors determining the protective activity of
chemopreventive agents against AFB1 hepatocarcinogenesis
is their ability to increase the biotransformation of AFB1
into non-toxic products. The mechanisms that mediate these
anticarcinogenic activities against AFB1-induced
hepatocarcinogenesis have been extensively studied for various
compounds such as EQ, oltipraz, BHT, beta-naphthoflavone (BNF)
and I3C (7,9,12,42–44).
Primary detoxification of AFB1 could be achieved through
the increased formation of AFM1 and AFQ1.
The increase of AFM1 formation through CYP1A1/2
induction appears to be a relevant mechanism for the protection
conferred by I3C, BNF and canthaxanthin against AFB1
in rats (37,42,45).
In the same manner, the enhancement of AFQ1 production
related to the induction of CYP2B1/2 and to a lesser extent of
CYP3A1/2 contributes to the anticarcinogenic activity of BHT and
I3C (7,42).
Another important resistance mechanism against AFB1 is
the increased detoxification of AFBO via rGSTA5 induction (8,9,11).
The induction of rGSTA5 appears to be a significant mechanism
responsible for the anticarcinogenic activity of oltipraz, EQ,
coumarin and I3C (9,11,13).
In addition, AFAR is probably involved in the resistance to AFB1
(11,15).
Different mechanisms can therefore contribute to the protective
activity of agents against deleterious effects of AFB1.
In order to identify the mechanisms responsible for the chemoprotective properties of garlic compounds, we evaluated the ability of liver subcellular fractions from DAS- and DADS-treated rats to modulate AFB1 mutagenesis in the Ames test. This approach allowed us to investigate the incidence of in vivo modulation of phase I and phase II enzymes by sulfur compound treatments. We showed that AFB1 microsome-mediated mutagenesis was inhibited by DAS treatment. This suggests that DAS could exert a protective effect against AFB1 by modulation of the oxidative metabolism of AFB1. DAS induced the formation of both the genotoxic metabolite of AFB1, AFB1-epoxide (measured as Tris-diol) and non-genotoxic metabolites, AFQ1 and AFM1. The formation of AFQ1 and AFM1 probably counterbalances the increase of AFB1-epoxide because microsomes from DAS-treated rats were shown to reduce AFB1 mutagenicity. Metabolism of AFB1 to epoxide is mainly attributed to CYP2C11 and to CYP3A2 in rat (5,6), while formation of AFQ1 can be attributed to the CYP2B and CYP3A families (7,42). DAS produced a strong induction of CYP2B1/2 and CYP3A2 this is in accordance with the increased levels of epoxide and AFQ1. It is not clear which isoenzyme is responsible for the increase in AFM1 formation as DAS showed no effect on CYP1A expression. Such a contradiction has also been reported for other chemopreventive compounds such as BHT (7). In contrast, DADS did not alter AFB1 microsome-mediated mutagenicity. DADS showed the same pattern of CYP induction as DAS but the level of induction detected by western blotting was less marked, especially for CYP2B1 and CYP3A2. Therefore, the induction of CYP2B and CYP3A2 provoked by DADS seems to be insufficient to significantly reduce the mutagenicity of AFB1.
Several lines of evidence suggest that the detoxification of AFBO through GSH conjugation is a key mechanism in reducing AFB1-induced hepatocarcinogenesis in rats (8,46,47). In this study, we demonstrated that DAS and DADS treatments resulted in effective inhibition of AFBO-induced mutagenesis. This effect is consistent with the increase of AFB1–GSH formation and is strongly related to the induction of rGSTA5 protein. The capacity of DADS to induce rGSTA5, to enhance AFB1–GSH conjugating activity as well as inhibit AFBO-induced mutagenesis was stronger than that of DAS. Interestingly, the effects of DADS were similar to those of the rGSTA5-model inducer, EQ. Our results are consistent with previous studies showing that DAS is a slight inducer of rGSTA5 (11) and that DAS and DADS stimulated GSTA5 gene expression (32). In contrast, administration of garlic oil to rats failed to induce rGSTA5 in rat liver (7). However, comparison with our results was not possible due to a lack of information on the composition of garlic oil administered in this study. Evidence for the involvement of rGSTA5 in the antimutagenic activity of DAS and DADS against AFBO was demonstrated by an immunoinhibition study with antibodies raised against rGSTA5. The subunit rGSTA5 inactives with high affinity the exo-AFBO (9,46), which is 500-fold more mutagenic than the endo-AFBO in the Ames test (4). However, pre-incubation with antibodies raised against rGSTA5 was ineffective in totally suppressing the antimutagenic effect of DAS and DADS. It was demonstrated that rGSTM1 and M2, two of the major GST subunits in the rat liver (48), have some abilities to conjugate the exo-AFBO (46,47). As DAS and DADS are effective inducers of enzyme activity related to mu class GST (33) and of protein expression of rGSTM1 and M2 (30), the induction of mu GST subunits might account for some antimutagenic activity of DAS and DADS against AFBO.
In a previous experiment, we demonstrated that DADS and DAS inhibited AFB1-induced hepatocarcinogenesis in the rat (25). DADS was shown to give complete protection against AFB1 as it reduced the number of pre-neoplastic lesions by >95% whereas DAS decreased this number by only 50%. The results presented in this report are consistent with these protective effects. Indeed, the effectiveness of DAS and DADS in inhibiting AFB1-induced pre-neoplastic lesions is correlated with their ability to inhibit AFBO-mediated mutagenicity: in both studies, DADS was more efficient than DAS. DAS demonstrated a dual protective mechanism, i.e. an inhibition of the activation of AFB1 and an enhancement of the detoxification of AFBO. DADS prevented AFB1 mutagenicity only by enhancing AFBO detoxification, but to a greater extent than DAS. Therefore, the induction of rGSTA5 appears to be the most relevant mechanism for the anticarcinogenic activity of garlic sulfur compounds.
Furthermore, in this study, we demonstrated that treatment with sulfur compounds induced a marked increase in the level of rAFAR1 protein, which is involved in the detoxification of AFB1 (15). DADS was shown to be more effective in inducing rAFAR1 than DAS. To our knowledge, this is the first demonstration that garlic sulfur compounds can influence the expression of this aldehyde reductase. The overexpression of GSTA5 and AFAR1 could be responsible for the chemopreventive properties of sulfur compounds against AFB1 carcinogenesis, by reducing the genotoxicity and the cytotoxicity of AFB1, respectively. These effects could explain the reduction in the number and the size of AFB1-induced preneoplastic foci that we have reported previously (25).
This study showed that DADS and EQ possess a similar pattern of induction. Like EQ, DADS is a potent inducer of several detoxification enzymes such as NQO, GST, UGT, EH and AFAR in rat liver (26,28,30,31). DADS and EQ show the same profile of GST induction since both induce rGSTA5 and rGSTP1, which are involved in the detoxification of chemical carcinogens (7,12). Therefore, DADS seems to be as efficient as EQ but it would be interesting to study the dose effect relationship of these two compounds when administered via the same route.
In conclusion, this study demonstrated that protection against AFB1 carcinogenesis conferred by DAS and DADS can be related to the modulation of enzymes involved in the metabolism of AFB1. The major determinant in this chemoprotective activity appears to be the induction of rGSTA5. DADS was shown to greatly reduce AFB1 genotoxicity by enhancing AFBO detoxification. This compound was also found to markedly reduce the mutagenicity of other chemical carcinogens (33,34). Together, these results demonstrate that DADS can prevent carcinogenesis induced by a broad range of environmental carcinogens. Moreover, the protective activity of DAS and DADS is not restricted to the initiation phase since they are able to inhibit the proliferation of tumor cells (18,49). The ability of garlic compounds to modify cellular events involved in the initiation and promotion steps of carcinogenesis suggests that consumption of garlic should enhance the resistance of humans to carcinogenesis. Garlic consumption could be therefore an attractive strategy for chemoprevention in individuals who are exposed to dietary aflatoxins. Until now, the effects of garlic or its constituents on cancer-related biomarkers have not been studied in humans. Induction of intestinal GST has been shown to occur in rats at doses of DADS similar to those that could be attained in human diet through the consumption of garlic (31). Additional investigations must be done to determine if the protective effects demonstrated in animal models are relevant for human.
|
|
Notes |
|---|
1 To whom correspondence should be addressed
Email:
lebon@dijon.inra.fr
![]()
|
|
Acknowledgments |
|---|
We thank Marie-France Vernevaut and Lucien Guenot (INRA, Dijon,
France) for excellent technical assistance. This work was supported
by funds from the Conseil Régional de Bourgogne.
|
|
References |
|---|
|
|
|---|
- Eaton,D.L. and Gallagher,E.P. (1994) Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol., 34, 135–172.[Medline]
- Groopman,J.D., Cain,L.G. and Kensler,T.W. (1988) Aflatoxin exposure in human populations: measurements and relationship to cancer. Crit. Rev. Toxicol., 19, 113–145.[Medline]
- Qian,G.S., Ross,R.K., Yu,M.C., Yuan,J.M., Gao,Y.T., Henderson,B.E., Wogan,G.N. and Groopman,J.D. (1994) A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol., Biomarkers Prev., 3, 3–10.[Abstract]
- Iyer,R.S., Coles,B.F., Raney,K.D., Thier,R., Guengerich,F.P. and Harris,T.M. (1994) DNA adduction by the potent carcinogen aflatoxin B1: mechanistic studies. J. Am. Chem. Soc., 116, 1603–1609.
- Shimada,T., Nakamura,S., Imaoka,S. and Funae,Y. (1987) Genotoxic and mutagenic activation of aflatoxin B1 by constitutive forms of cytochrome P-450 in rat liver microsomes. Toxicol. Appl. Pharmacol., 91, 13–21.[Medline]
- Buetler,T.M., Bammler,T.K., Hayes,J.D. and Eaton,D.L. (1996) Oltipraz-mediated changes in aflatoxin B1 biotransformation in rat liver: implications for human chemointervention. Cancer Res., 56, 2306–2313.[Abstract]
- Manson,M.M., Ball,H.W., Barrett,M.C., Clark,H.L., Judah,D.J., Williamson,G. and Neal,G.E. (1997) Mechanism of action of dietary chemoprotective agents in rat liver: induction of phase I and II drug metabolizing enzymes and aflatoxin B1 metabolism. Carcinogenesis, 18, 1729–1738.[Abstract]
- Hayes,J.D., Judah,D.J., McLellan,L.I. and Neal,G.E. (1991) Contribution of the glutathione S-transferases to the mechanisms of resistance to aflatoxin B1. Pharmacol. Ther., 50, 443–472.[Medline]
- Hayes,J.D., Judah,D.J., McLellan,L.I., Kerr,L.A., Peacock,S.D. and Neal,G.E. (1991) Ethoxyquin-induced resistance to aflatoxin B1 in the rat is associated with the expression of a novel alpha-class glutathione S-transferase subunit, Yc2, which possesses high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem. J., 279, 385–398.[Medline]
- Hayes,J.D.,
Nguyen,T., Judah,D.J., Petersson,D.G. and Neal,G.E. (1994) Cloning of
cDNAs from fetal rat liver encoding glutathione S-transferase Yc
polypeptides. The Yc2 subunit is expressed in adult rat liver resistant to
the hepatocarcinogen aflatoxin B1. J. Biol. Chem.,
269, 20707–20717.
[Abstract/Free Full Text] - Hayes,J.D., Mcleod,R., Ellis,E.M., Pulford,D.J., Ireland,L.S., McLellan,L.I., Judah,D.J., Manson,M.M. and Neal,G.E. (1996) Regulation of glutathione S-transferases and aldehyde reductase by chemoprotectors: studies of mechanisms responsible for inducible resistance to aflatoxin B1. In Steward,B.W., McGregor,D. and Kleihues,P. (eds) Principles of Chemoprevention. IARC Scientific Publications No. 139, IARC, Lyon, pp. 175–187.
- Kelly,V.P.,
Ellis,E.M., Manson,M.M., Chanas,S.A., Moffat,G.J., Mcleod,R., Judah,D.J.,
Neal,G.E. and Hayes,J.D. (2000) Chemoprevention of aflatoxin B1
hepatocarcinogenesis by coumarin, a natural benzopyrone that is a potent
inducer of aflatoxin B1-aldehyde reductase, the glutathione
S-transferase A5 and P1 subunits and NAD (P)H:quinone oxidoreductase
in rat liver. Cancer Res., 60, 957–969.
[Abstract/Free Full Text] - Stresser,D.M., Williams,D.E., McLellan,L.I., Harris,T.M. and Bailey,G.S. (1994) Indole-3-carbinol induces a rat liver glutathione transferase subunit (Yc2) with high activity toward aflatoxin B1 exo-epoxide. Association with reduced levels of hepatic aflatoxin–DNA adducts in vivo. Drug Metab. Disp., 22, 392–399.[Abstract]
- Hayes,J.D., Judah,D.J., Neal,G.E. and Nguyen,T. (1992) Molecular cloning and heterologous expression of a cDNA encoding a mouse glutathione S-transferase Yc subunit possessing high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem. J., 285, 173–180.[Medline]
- Hayes,J.D., Judah,D.J. and Neal,G.E. (1993) Resistance to aflatoxin B1 is associated with the expression of a novel aldo-keto reductase which has catalytic activity towards a cytotoxic aldehyde-containing metabolite of the toxin. Cancer Res., 53, 3887–3894.[Abstract]
- Ellis,E.M., Judah,D.J., Neal,G.E., O’Connor,T. and Hayes,J.D. (1996) Regulation of carbonyl-reducing enzymes in rat liver by chemoprotectors. Cancer Res., 56, 2758–2766.[Abstract]
-
Fleischauer,A.T. and Arab,L. (2001) Garlic and cancer: a critical review
of the epidemiologic literature. J. Nutr., 131, 1032S–1040S.
[Abstract/Free Full Text] - Le Bon,A.M. and Siess,M.H. (2000) Organosulfur compounds from Allium and the chemoprevention of cancer. Drug Metab. Drug Interact., 17, 51–79.[Medline]
- Sparnins,V.L., Barany,G. and Wattenberg,L.W. (1988) Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis, 9, 131–134.[Abstract]
- Wargovich,M.J. (1987) Diallyl sulfide, a flavor component of garlic (Allium sativum), inhibits dimethylhydrazine-induced colon cancer. Carcinogenesis, 8, 487–489.[Abstract]
- Wattenberg,L.W., Sparnins,V.L. and Barany,G. (1989) Inhibition of N-nitrosodiethylamine carcinogenesis in mice by naturally occurring organosulfur compounds and monoterpenes. Cancer Res., 49, 2689–2692.[Abstract]
- Ip,C., Lisk,D.J. and Stoewsand,G.S. (1992) Mammary cancer prevention by regular garlic and selenium-enriched garlic. Nutr. Cancer, 17, 279–286.[Medline]
- Hong,J.Y., Wang,Z.Y., Smith,T.J., Zhou,S., Shi,S., Pan,J. and Yang,C.S. (1992) Inhibitory effects of diallyl sulfide on the metabolism and tumorigenicity of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A/J mouse lung. Carcinogenesis, 13, 901–904.[Abstract]
- Surh,Y.J., Lee,R.C., Park,K.K., Mayne,S.T., Liem,A. and Miller,J.A. (1995) Chemoprotective effects of capsaicin and diallyl sulfide against mutagenesis or tumorigenesis by vinyl carbamate and N-nitrosodimethylamine. Carcinogenesis, 16, 2467–2471.[Abstract]
- Haber-Mignard,D., Suschetet,M., Berges,R., Astorg,P. and Siess,M.H. (1996) Inhibition of aflatoxin B1- and N-nitrosodiethylamine-induced liver preneoplastic foci in rats fed naturally occurring allyl sulfides. Nutr. Cancer, 25, 61–70.[Medline]
- Siess,M.H., Le Bon,A.M., Canivenc-Lavier,M. and Suschetet,M. (1997) Modification of hepatic drug-metabolizing enzymes in rats treated with alkyl sulfides. Cancer Lett., 120, 195–201.[Medline]
- Haber,D., Siess,M.H., Canivenc-Lavier,M.C., Le Bon,A.M. and Suschetet,M. (1995) Differential effects of dietary diallyl sulfide and diallyl disulfide on rat intestinal and hepatic drug-metabolizing enzymes. J. Toxicol. Environ. Health, 44, 423–434.[Medline]
- Haber,D., Siess,M.H., de Waziers,I., Beaune,P. and Suschetet,M. (1994) Modification of hepatic drug-metabolizing enzymes in rat fed naturally occurring allyl sulphides. Xenobiotica, 24, 169–182.[Medline]
- Brady,J.F., Li,D.C., Ishizaki,H. and Yang,C.S. (1988) Effect of diallyl sulfide on rat liver microsomal nitrosamine metabolism and other monooxygenase activities. Cancer Res., 48, 5937–5940.[Abstract]
- Guyonnet,D., Siess,M.H., Le Bon,A.M. and Suschetet,M. (1999) Modulation of phase II enzymes by organosulfur compounds from allium vegetables in rat tissues. Toxicol. Appl. Pharmacol., 154, 50–58.[Medline]
- Munday,R. and Munday,C.M. (1999) Low doses of diallyl disulfide, a compound derived from garlic, increase tissue activities of quinone reductase and glutathione transferase in the gastrointestinal tract of the rat. Nutr. Cancer, 34, 42–48.[Medline]
- Kim,S.G., Chung,H.C. and Cho,J.Y. (1996) Molecular mechanism for alkyl sulfide-modulated carbon tetrachloride-induced hepatotoxicity: the role of cytochrome P450 2E1, P450 2B and glutathione S-transferase expression. J. Pharmacol. Exp. Ther., 277, 1058–1066.[Abstract]
- Guyonnet,D., Belloir,C., Suschetet,M., Siess,M.H. and Le Bon,A.M. (2001) Antimutagenic activity of organosulfur compounds from Allium is associated with phase II enzyme induction. Mutat. Res., 495, 135–145.[Medline]
- Guyonnet,D., Belloir,C., Suschetet,M., Siess,M.H. and LeBon,A.M. (2000) Liver subcellular fractions from rats treated by organosulfur compounds from Allium modulate mutagen activation. Mutat. Res., 466, 17–26.[Medline]
- Bradford,M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72, 248–254.[Medline]
- Teyssier,C.,
Guenot,L., Suschetet,M. and Siess,M.H. (1999) Metabolism of diallyl
disulfide by human liver microsomal cytochromes P-450 and flavin-containing
monooxygenases. Drug Metab. Disp., 27, 835–841.
[Abstract/Free Full Text] - Gradelet,S., Le Bon,A.M., Berges,R., Suschetet,M. and Astorg,P. (1998) Dietary carotenoids inhibit aflatoxin B1-induced liver preneoplastic foci and DNA damage in the rat: role of the modulation of aflatoxin B1 metabolism. Carcinogenesis, 19, 403–411.[Abstract]
- Neal,G.E., Judah,D.J., Stirpe,F. and Patterson,D.S. (1981) The formation of 2,3-dihydroxy-2,3-dihydro-aflatoxin B1 by the metabolism of aflatoxin B1 by liver microsomes isolated from certain avian and mammalian species and the possible role of this metabolite in the acute toxicity of aflatoxin B1. Toxicol. Appl. Pharmacol., 58, 431–437.[Medline]
- Tsikas,D. and Brunner,G. (1999) High-performance liquid chromatography of glutathione conjugates. Fresen. J. Anal. Chem., 343, 330–334.
- Kelly,V.P., Ireland,L.S., Ellis,E.M. and Hayes,J.D. (2000) Purification from rat liver of a novel constitutively expressed member of the aldo-keto reductase 7 family that is widely distributed in extrahepatic tissues. Biochem. J., 348, 389–400.[Medline]
- Maron,D.M. and Ames,B.N. (1983) Revised methods for the Salmonella mutagenicity test. Mutat. Res., 113, 173–215.[Medline]
- Stresser,D.M., Bailey,G.S. and Williams,D.E. (1994) Indole-3-carbinol and beta-naphthoflavone induction of aflatoxin B1 metabolism and cytochromes P-450 associated with bioactivation and detoxication of aflatoxin B1 in the rat. Drug Metab. Disp., 22, 383–391.[Abstract]
- Kensler,T.W., Egner,P.A., Dolan,P.M., Groopman,J.D. and Roebuck,B.D. (1987) Mechanism of protection against aflatoxin tumorigenicity in rats fed 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (oltipraz) and related 1,2-dithiol-3-thiones and 1,2-dithiol-3-ones. Cancer Res., 47, 4271–4277.[Abstract]
- Kensler,T.W., Groopman,J.D., Sutter,T.R., Curphey,T.J. and Roebuck,B.D. (1999) Development of cancer chemopreventive agents: oltipraz as a paradigm. Chem. Res. Toxicol., 12, 113–126.[Medline]
- Gurtoo,H.L., Koser,P.L., Bansal,S.K., Fox,H.W., Sharma,S.D., Mulhern,A.I. and Pavelic,Z.P. (1985) Inhibition of aflatoxin B1-hepatocarcinogenesis in rats by beta-naphthoflavone. Carcinogenesis, 6, 675–678.[Abstract]
- Johnson,W.W., Ueng,Y.F., Widersten,M., Mannervik,B., Hayes,J.D., Sherratt,P.J., Ketterer,B. and Guengerich,F.P. (1997) Conjugation of highly reactive aflatoxin B1 exo-8,9-epoxide catalyzed by rat and human glutathione transferases: estimation of kinetic parameters. Biochemistry, 36, 3056–3060.[Medline]
- Raney,K.D., Meyer,D.J., Ketterer,B., Harris,T.M. and Guengerich,F.P. (1992) Glutathione conjugation of aflatoxin B1 exo- and endo-epoxides by rat and human glutathione S-transferases. Chem. Res. Toxicol., 5, 470–478.[Medline]
- Hayes,J.D. and Pulford,D.J. (1995) The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol., 30, 445–600.[Medline]
- Knowles,L.M. and Milner,J.A. (2000) Allyl sulfides modify cell growth. Drug Metab. Drug Interact., 17, 81–107.[Medline]
Received August 8, 2001; revised April 22, 2002; accepted April 23, 2002.