|
Mycotoxins, by-products of fungal metabolism, have been implicated
as causative agents of adverse health effects in humans and animals that
have consumed fungus-infected agricultural products.1,2
Consequently, fungi that produce mycotoxins, as well as the mycotoxins
themselves, are potential problems from both public health and economic
perspectives. The fungi are a vast assemblage of living organisms, but
mycotoxin production is most commonly associated with the terrestrial
filamentous fungi called the molds.3 Various genera of
toxigenic fungi are capable of producing such diverse mycotoxins as the
aflatoxins, rubratoxins, ochratoxins, fumonisins, and trichothecenes.1,2
The trichothecenes are a very large family of chemically related
toxins produced by various species of Fusarium, Myrotecium,
Trichoderma, Cephalosporium, Verticimonosporium, and Stachybotrys.4
They are markedly stable under different environ mental conditions. The
distinguishing chemical feature of trichothecenes is the presence of a
trichothecene ring, which contains an olefinic bond at C-9, 10; and an
epoxide group at C-12, 12.5 All trichothecenes are
mycotoxins, but not all mycotoxins are trichothecenes. This family of
mycotoxins causes multiorgan effects including emesis and diarrhea,
weight loss, nervous disorders, cardiovascular alterations,
immunodepression, hemostatic derangements, skin toxicity, decreased
reproductive capacity, and bone marrow damage.4,6
In this chapter, we will concentrate on T-2 mycotoxin, a highly
toxic trichothecene that, together with some closely related compounds,
has been the causative agent of a number of illnesses in humans and
domestic animals.1,2,4 During the 1970s and 1980s, the
trichothecene mycotoxins gained some notoriety as putative biological
warfare agents when they were implicated in “yellow rain” attacks in
Southeast Asia.7–11
Fungi that produce trichothecenes are plant pathogens and invade
various agricultural products and plants. Since Fusarium and
other related fungi infect important foodstuff, they have been
associated worldwide with intoxication of humans and animals. Thus,
these fungi have potential as biological weapons.
Use in Biological Warfare
From 1974 to 1981, toxic agents were used by the Soviet Union and
its client states in such Cold War sites as Afghanistan, Laos, and
Kampuchea (Cambodia). Aerosol-and-droplet clouds were produced by
delivery systems in the Soviet arsenal such as aircraft spray tanks,
aircraft-launched rockets, bombs (exploding cylinders), canisters, a
Soviet hand-held weapon (DH-10), and booby traps. Aircraft used for
delivery included L-19s, AN-2s, T-28s, T-41s, MiG-21s (in Laos) and
Soviet MI-24 helicopters (in Afghanistan and Laos).
Attacks in Laos (1975–1981) were directed against Hmong villagers
and resistance forces who opposed the Lao People’s Liberation Army and
the North Vietnamese. In Kampuchea, North Vietnamese troops used 60-mm
mortar shells; 120-mm shells; 107-mm rockets; M-79 grenade launchers
containing chemicals; and chemical rockets, bombs, and sprays delivered
by T-28 aircraft (1979–1981) against Khmer Rouge troops. The chemical
munitions were supplied by the Soviets and delivered by North Vietnamese
or Laotian pilots. In Afghanistan, the chemical weapons were delivered
by Soviet or Afghan pilots against Mujahidin guerrillas (1979–1981).
Lethality of the attacks is documented by a minimum of 6,310 deaths in
Laos (from 226 attacks); 981 deaths in Kampuchea (from 124 attacks); and
3,042 deaths in Afghanistan (from 47 attacks).7
Trichothecenes appear to have been used in some of these attacks.
The air attacks in Laos have been described as “yellow rain” and
consisted of a shower of sticky, yellow liquid that sounded like rain as
it fell from the sky. Other accounts described a yellow cloud of dust or
powder, a mist, smoke, or insect spray–like material. Liquid agent
rapidly dried to a powder. In Laos, 50% to 81%7 of attacks
involved material associated with a yellow pigment. Other attacks were
associated with red, green, white, or brown smoke or vapor. More than
80%7 of attacks were delivered by air-to-surface rockets; the
remainder, from aircraft spray tanks or bombs. Intelligence information
and some of the victims’ descriptions of symptoms raised the possibility
that chemical warfare agents such as phosgene, sarin, soman, mustards,
CS, phosgene oxime, or BZ may also have been used. These agents may have
been used in mixtures or alone, and with or without the trichothecenes.
Unconfirmed reports have implicated the use of trichothecenes in
the 1964 Egyptian (or Russian) attacks on Yemeni Royalists in Yemen12
and in combination with mustards during chemical warfare attacks in the
Iran–Iraq War (1983–1984).13 According to European sources,
Soviet–Cuban forces in Cuba are said to have been equipped with
mycotoxins, and a Cuban agent is said to have died of a hemorrhagic
syndrome induced by a mycotoxin agent.14
|
The
Yellow Rain Controversy
Actual biological warfare use of trichothecenes in Southeast Asia
and Afghanistan is strongly supported by the epidemiological and
intelligence assessments and trichothecene assays, although reports in
the open literature have discounted this contention. An article written
by L. R. Ember,15 published in 1984 in Chemical Engineering
News, is the most exhaustive and authoritative account of the
controversy surrounding the use of trichothecene mycotoxins in Southeast
Asia during the 1970s.
The United States government, its allies, and journalists
exhaustively studied the possibility that yellow rain attacks had
occurred, based on evidence7,14,15 such as the following:
- interviews of Hmong
survivors of and eyewitnesses to lethal yellow rain attacks in Laos,
who provided consistent descriptions of the episodes;
- interrogations of a
defecting Laotian Air Force officer and North Vietnamese ground
troops, who corroborated the descriptions of attacks and admitted
using the chemicals;
- interrogations of
prisoners of war, who admitted being involved in attacks where
unconventional weapons were used (ie, in Afghanistan);
- laboratory
confirmations of Soviet use of chemical agents, and
- the presence of
Soviet-manufactured chemical agents and Soviet technicians in Laos.
The evidence supports
the contention that trichothecene mycotoxins were used as biological
warfare agents in Southeast Asia and Afghanistan by the former Soviet
Union and its surrogates. The Russians have not recently denied such use
but have declined to discuss the subject.
In addition to the evidence stated above, elevated levels and
naturally rare mixtures of trichothecene toxins were recovered from the
surfaces of plants, fragments of plastic, and rocks in areas attacked9,11,15,16
; and were detected in the blood of attack survivors and the tissues of
a dead casualty.10,15 Control samples that were taken (a)
from an environment that had not been attacked, and during another
season of the year,15 and (b) from Hmong who had never
been exposed to an attack were consistently negative.
The evidence that trichothecenes were used in Southeast Asia has
been challenged: questions have been raised about the interview
methodology used by U.S. Army physicians and U.S. State Department
personnel in Hmong refugee camps in Thailand to obtain descriptions of
the attacks. Some inconsistencies of specific individuals’ stories were
demonstrated, but the frequency of unreliable information has not been
reported and is unlikely to be large enough to discredit all witnesses.15
Symptom descriptions are generally consistent with known trichothecene
effects.
The paucity of positive evidence of the presence of trichothecenes
(5 positive environmental and 20 positive biomedical samples) has been
used to challenge the belief that biological warfare attacks occurred,
since only 10% of samples were positive. However, 32% of samples from
victims were positive, a value too high for natural causes (eg, food
contamination) to be used as an explanation, since 98% of controls in
nonattack areas of Thailand were negative.17 The 2% of
samples that were positive could represent either a nonspecific result
or low-prevalence food contamination. The paucity and type of control
samples have also been questioned.
Some experts18–21 have claimed that yellow rain was not
a biological warfare attack at all, but that the yellow residue was
caused by showers and deposits of bee feces—the result of massive bee
swarming and cleansing–defecation flights over some areas of Southeast
Asia. The presence of pollen in bee feces and some samples has not only
added confusion18 but is also the supporting evidence used by
the skeptics. It is important to remember that persons caught in a
shower of bee feces do not get sick and die. Although bee flights have
occurred before and since 1982, reports of attacks of yellow rain and
death in Asia have not.
Then what explains the symptoms consistent with trichothecene
effects in the casualties, and the pollen and bee feces in some of the
yellow spots on vegetation in the area? Bee feces do not contain
trichothecenes, yet pollen and trichothecenes without mold are found
together in some samples from attack areas. The most likely explanation
is that during biological warfare attacks, dispersed trichothecenes
landed in pollen-containing areas.
French scientists have reported the simultaneous synthesis of three
trichothecene toxins by Fusarium growing on corn, but actual
production of these toxins by Fusarium species in Southeast Asia
has not been demonstrated, presumably because of high environmental
temperature (ie, toxin production usually increases at low
temperatures). Whether or not Fusarium toxin is produced in the
high-mountain temperate regions of Laos inhabited by the Hmong remains
unanswered. The presence of toxin on leaves without accompanying mold
also is unexplained by critics of the trichothecene weapon hypothesis.
In vivo studies have demonstrated that F semitectum var
semitectum will grow on leaves in Southeast Asia, but have not shown
that it will produce toxin in vivo.15
In support of the weapon hypothesis are the positive trichothecene
analyses performed by two leading researchers9,10 in the
detection of trichothecenes; the Defense Research Establishment, Ottawa,
Canada11,22; and the U.S. Army Chemical Research and
Development Center, Edgewood, Maryland.23 Negative results of
analyses of biomedical and environmental samples from Southeast Asia
have come from Porton Down Laboratory in England,17,24 but
according to the British, such results do not exclude sampling problems,
including delay in sample collection after an attack, as a cause of the
negative results.15
Proponents have been accused of analyzing samples that were
purposely contaminated with toxin, either after collection or during the
analysis. Other methodological criticisms include poor recovery (< 10%
of one sample spiked with T-2 toxin); low precision of quantitative data
when analyzing two portions of the same leaf; and lack of
well-documented, confirming, replicate analyses in Mirocha’s or a
similarly equipped second laboratory.15 The presence of
polyethylene glycol in the sample analyzed by Rosen9 also
indicates that the trichothecene mixture detected was manufactured, not
natural.
Many experts in the intelligence community,16 academia,8,9
the U.S. Department of State,7 and the authors of this
chapter believe that trichothecenes were used as biological weapons in
Southeast Asia and Afghanistan. However, a weapon containing
trichothecenes was not found in Southeast Asia, and the Soviets have not
declared any stockpiles of trichothecenes among their chemical or
biological weapons. Thus, it has not been possible for the United States
to prove unequivocally that trichothecene mycotoxins were used as
biological weapons.
Weaponization
Trichothecene mycotoxins can be delivered as dusts, droplets,
aerosols, or smoke from aircraft, rockets, missiles, artillery, mines,
or portable sprayers. Because of their antipersonnel properties, ease of
large-scale production, and apparent proven delivery by various aerial
dispersal systems, the trichothecene mycotoxins (especially T-2 toxin)
have an excellent potential for weaponization.
When delivered at low doses, trichothecene mycotoxins cause skin,
eye, and gastrointestinal problems. In nanogram amounts,4,25
they (T-2 toxin, in particular) cause severe skin irritation (erythema,
edema, and necrosis).4,6 Skin vesication has been observed in
a number of humans exposed to yellow rain attacks.4,14,15 T-2
toxin is about 400-fold more potent (50 ng vs 20 µg) than mustard in
producing skin injury.26 Lower-microgram quantities of
trichothecene mycotoxins cause severe eye irritation, corneal damage,
and impaired vision.4,16,26,27 Emesis and diarrhea have been
observed at amounts that are one fifth to one tenth the lethal doses of
trichothecene mycotoxins.26
Depending on the species of experimental animal tested and the
exposure procedure,28,29 the lethality of T-2 toxin by
aerosol exposure can be 10- to 50-fold greater than when injected
parenterally.30 With larger doses in humans, aerosolized
trichothecenes may produce death within minutes to hours.7,14,15
The term LCt50 (the concentration • time that is
lethal to 50% of the exposed population) is used to describe exposure to
vapors and aerosols; milligrams • minutes per cubic meter is the
conventional unit of measurement. LCt50 and its
relation to LD50 (the dose that is lethal to 50% of the
exposed population) are discussed in detail in
Chapter 5, Nerve
Agents, and will not be further explicated here.
The toxicity of T-2 toxin by the inhalational route of exposure (LCt50
range: 200–5,800 mg•min/m3 ) 28–30 is similar to
that observed for mustards or Lewisite (LCt50 range:
1,500–1,800 mg•min/m3). 31 However, the lethality
of T-2 toxin by the dermal route (LD50 range: 2–12 mg/kg6)
is higher than that for liquid Lewisite (LD50: approximately
30 mg/kg31(p39)) or liquid mustards (LD50:
approximately 100 mg/kg31(p32)). Therefore, the trichothecene
mycotoxins are considered to be primarily blister agents that, at lower
exposure concentrations, can cause severe skin and eye irritation, and
at larger doses can produce considerable incapacitation and death within
minutes to hours.
By solid substrate fermentation, T-2 toxin can be produced at
approximately 9 g/kg of substrate, with a yield of 2 to 3 g of
crystalline product.32 Several of the trichothecene
mycotoxins have been produced in liquid culture at medium yields and
large volumes of culture for extraction.33 Thus, using
existing state-of-the-art fermentation processes that were developed for
brewing and antibiotics, it would be fairly simple to produce ton
quantities of a number of the trichothecene mycotoxins.
In Southeast Asia, most of the yellow rain attacks were delivered
by aircraft or helicopter spray, bombs, and air-to-surface rockets. The
attacks were described as a shower of sticky liquid, a yellow cloud of
dust or powder, or a mist (like an insect spray).7,15 The
delivery of the trichothecene mycotoxins was similar in many aspects to
the spraying of pesticides on agricultural crops. This would result in a
very low-efficiency respiratory aerosol (1–5 µm particles)34
but a highly effective droplet aerosol that could cause severe skin and
eye irritation. |
|
Occurrence in Nature
Potentially hazardous concentrations of the trichothecene
mycotoxins can occur naturally in moldy grains, cereals, and
agricultural products.4,35 Toxigenic species of Fusarium
occur worldwide in habitats as diverse as deserts, tidal salt flats, and
alpine mountain regions.35 For example, a food-related
disease has been recorded in Russia from time to time, probably since
the 19th century.36 Over the period 1942 through 1947, more
than 10% of the population in Orenburg, near Siberia, were fatally
affected by overwintered millet, wheat, and barley.4,36 The
syndrome was officially named alimentary toxic aleukia (ATA). Extensive
investigations in Russia indicated that a toxin from Fusarium
species of fungi was the causative agent of ATA.36,37
Subsequently, it was demonstrated that T-2 toxin, a potent trichothecene
mycotoxin, was the likely agent.37
Stachybotryotoxicosis has been reported among farm workers in
Russia, Yugoslavia, and Hungary.38,39 This disease is caused
by the presence of a mold, Stachybotrys atra (S alternans), on
the hay fed to domestic animals. A macrocyclic trichothecene (satratoxin)
produced by the Stachybotrys species of the mold may be in part
responsible for this toxicosis.40 The only literature
citation on apparent human cases of stachybotryotoxicosis in the United
States occurred in people living in a water-damaged house with a heavy
infestation of S atra.41
Russian scientists have reported a case of “cotton lung disease,”
which was brought about by the inhalation of cotton dust that was
contaminated with Dendrochium toxicum. This fungus is considered
to be synonymous with Myrothecium verrucaria (a natural producer
of the verrucarin class of trichothecenes).42
The “red mold disease” of wheat and barley in Japan is prevalent in
the region that faces the Pacific Ocean.4 Toxic
trichothecenes, including nivalenol, deoxynivalenol, and
monoacetylnivalenol (fusarenon-X) from Fusarium nivale, can be
isolated from moldy grains. In the suburbs of Tokyo, an illness similar
to “red mold disease” was described in an outbreak of a food-borne
disease, as a result of the consumption of Fusarium-infected
rice.35 Ingestion of moldy grains that are contaminated with
trichothecenes has been associated with mycotoxicosis in domestic farm
animals.4
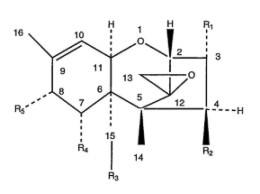 |
| Fig. 34-1.
The general structure, numbering system, and variable side groups of
the tetracyclic trichothecene nucleus. |
Chemical and Physical Properties
The trichothecenes make up a family of closely related chemical
compounds called sesquiterpenoids (Figure 34-1). The structures
of close to 150 derivatives of the trichothecenes are described in the
scientific literature.35,43 The specific side structures of
the most abundant of the naturally occurring trichothecenes are shown in
Table 34-1. Because of its availability and relatively high toxicity,
T-2 toxin has been the most extensively studied trichothecene mycotoxin.
The trichothecene mycotoxins are nonvolatile, low-molecular-weight
(MW 250–550) compounds.43 This group of mycotoxins is
relatively insoluble in water but highly soluble in acetone, ethyl
acetate, chloroform, dimethyl sulfoxide (DMSO), ethanol, methanol, and
propylene glycol.43 Purified trichothecenes generally have a
low vapor pressure, but they do vaporize when heated in organic
solvents. Extraction of trichothecene mycotoxins from fungal cultures
with organic solvents yields a yellow-brown liquid, which, if allowed to
evaporate, forms a greasy, yellow crystalline product. Some experts10,16
believe this extract to be the yellow contaminate of yellow rain. In
contrast, highly purified toxins form white, crystalline products that
have characteristic melting points.35
When maintained as either crystalline powders or liquid solutions,
trichothecene mycotoxin compounds are stable when exposed to air, light,
or both.35,44 Moreover, these mycotoxins are not inactivated
by autoclaving but require heating at 900°F for 10 minutes or 500°F for
30 minutes for complete inactivation. A 3% to 5% solution of sodium
hypochlorite is an effective inactivation agent for them.44
The efficacy of this solution can be increased by the addition of small
amounts of alkali.
|
TABLE 34-1
SPECIFIC SIDE GROUPS OF THE MOST ABUNDANT TRICHOTHECENE MYCOTOXINS
|
|
Trichothecene |
R 1 |
|
R 2 |
|
R 3 |
|
R 4 |
|
R 5 |
|
|
T-2 Toxin |
–OH |
|
–OCOCH3 |
|
–OCOCH3 |
|
–H |
|
–OCOCH2
CH(CH3)2 |
|
HT-2 Toxin |
–OH |
|
–OH |
|
–OCOCH3 |
|
–H |
|
–OCOCH2
CH(CH3)2 |
4,15-Diacetoxyscripenol
(DAS, also called anguidine) |
–OH |
|
–OCOCH3 |
|
–OCOCH3 |
|
–H |
|
–H |
|
Nivalenol |
–OH |
|
–OH |
|
–OH |
|
–OH |
|
=O |
|
Deoxynivalenol (DON) |
–OH |
|
–H |
|
–OH |
|
–OH |
|
=O |
|
Macrocyclics |
–H |
–O–R '–O– |
|
–H |
|
–H |
R ': Macrocyclic
ester or ester–ether bridge between carbons 4 and 15. The most
abundant macrocyclic trichothecenes are verrucarins, roridins, and
satratoxin H. Source for this statement: Jarvis BB. Macrocyclic
trichothecenes. In: Sharma RP, Salunkhe DK, eds. Mycotoxins and
Phytoalexins. Boca Raton, Fla: CRC Press; 1991: 361–421. |
|
|
The trichothecene mycotoxins are toxic to humans, other mammals,
birds, fish, a variety of invertebrates, plants, and eukaryotic cells in
general. The acute toxicity of the trichothecene mycotoxins varies
somewhat with the particular toxin and animal species studied (Table
34-2). Differences are noted among the various species in their
susceptibility to trichothecene mycotoxins, but they are small compared
with the divergence obtained by diverse routes of administration of the
toxins (Table 34-3). Once the trichothecene mycotoxins enter the
systemic circulation, regardless of the route of exposure, they affect
rapidly proliferating tissues.1,2,4,6,35,42,45
TABLE 34-2
RELATIVE ACUTE PARENTERAL TOXICITY OF THE MOST ABUNDANT
TRICHOTHECENE MYCOTOXINS
|
|
|
Mammals Tested |
|
|
|
|
|
Mouse |
Rat |
Guinea Pig |
Rabbit |
Cat |
Dog |
Pig |
Monkey |
|
|
|
|
Trichothecenes
Tested |
LD50
(mg/kg) |
|
|
T-2 Toxin |
5.2 (IV) |
0.9 (IV) |
1.0 (IV) |
1.0 (IM) |
< 0.5 (SC) |
— |
1.2 (IV) |
0.8 (IM) |
|
HT-2 Toxin |
9.0 (IP) |
— |
— |
— |
— |
— |
— |
— |
4,15-Diacetoxy-
scripenol (DAS) |
12.0 (IV) |
1.3 (IV) |
— |
1.0 (IV) |
— |
1.1 (IV) |
0.38 (IV) |
— |
|
Nivalenol |
6.3 (IV) |
— |
— |
— |
— |
— |
— |
— |
Deoxynivalenol
(DON) |
43 (SC) |
— |
— |
— |
— |
— |
— |
— |
|
Verrucarin A |
1.5 (IV) |
0.8 (IV) |
— |
0.54 (IV) |
— |
— |
— |
— |
|
Roridin A |
1.0 (IV) |
— |
— |
— |
— |
— |
— |
— |
|
Satratoxin H |
1.0 (IP) |
— |
— |
— |
— |
— |
— |
— |
Routes of
administration of the mycotoxin: IV: intravenous; IM:
intramuscular; SC: subcutaneous; IP: intraperitoneal
—: Not determined
Data sources: (1) Ueno Y. Trichothecene mycotoxins: Mycology,
chemistry, and toxicology. Adv Nut Res. 1989;3:301–353.
(2) Wannemacher RW Jr, Bunner DL, Neufeld HA. Toxicity of
trichothecenes and other related mycotoxins in laboratory
animals. In: Smith JE, Henderson RS, eds. Mycotoxins and
Animal Foods. Boca Raton, Fla: CRC Press; 1991: 499–552. (3)
Sharma RP, Kim Y-W. Trichothecenes. In: Sharma RP, Salunkhe DK,
eds. Mycotoxins and Phytoalexins. Boca Raton, Fla: CRC
Press; 1991: 339–359. (4) Jarvis BB. Macrocyclic trichothecenes.
In: Sharma RP, Salunkhe DK, eds. Mycotoxins and Phytoalexins.
Boca Raton, Fla: CRC Press; 1991: 361–421. |
|
TABLE 34-3
COMPARATIVE TOXICITY OF T-2 TOXIN BY VARIOUS ROUTES OF
ADMINISTRATION
|
|
|
Mammals Tested |
|
|
|
|
|
Mouse |
Rat |
Guinea Pig |
Rabbit |
Cat |
Pig |
Monkey |
|
|
|
|
Route of
Administration |
T-2 Toxin LD50
(mg/kg) |
|
|
Intravenous |
4.2–7.3 |
0.7–1.2 |
1.0–2.0 |
— |
— |
1.2 |
— |
|
Intraperitoneal |
5.2–9.1 |
1.3–2.6 |
— |
— |
— |
— |
— |
|
Subcutaneous |
2.1–3.3 |
0.6–2.0 |
1.0–2.0 |
— |
<0.5 |
— |
— |
|
Intramuscular |
— |
0.5–0.9 |
1.0 |
1.1 |
— |
— |
0.8 |
|
Intragastric |
9.6–10.5 |
2.3–5.2 |
3.1–5.3 |
— |
— |
— |
— |
|
Intranasal |
— |
0.6 |
— |
— |
— |
— |
— |
|
Intratracheal |
0.16 |
0.1 |
— |
— |
— |
— |
— |
|
Inhalational |
0.24 |
0.05 |
0.6–2.0 |
— |
— |
— |
— |
|
Intracerebral |
— |
0.01 |
— |
— |
— |
— |
— |
|
Dermal in DMSO |
6.6 |
4.3 |
2.2 |
10 |
— |
— |
>8.0 |
|
Dermal in Methanol |
— |
>380 |
>80 |
— |
— |
— |
— |
DMSO: dimethyl
sulfoxide
—: Not determined
Data sources: (1) Ueno Y. Trichothecene mycotoxins: Mycology,
chemistry, and toxicology. Adv Nut Res. 1989;3:301–353.
(2) Wannemacher RW Jr, Bunner DL, Neufeld HA. Toxicity of
trichothecenes and other related mycotoxins in laboratory
animals. In: Smith JE, Henderson RS, eds. Mycotoxins and
Animal Foods. Boca Raton, Fla: CRC Press; 1991: 499–552. (3)
Sharma RP, Kim Y-W. Trichothecenes. In: Sharma RP, Salunkhe DK,
eds. Mycotoxins and Phytoalexins. Boca Raton, Fla: CRC
Press; 1991: 339–359. |
|
Mechanism of Action
The trichothecene mycotoxins are cytotoxic to most eukaryotic
cells.46 A number of cytotoxicity assays have been developed
and include survival and cloning assays, measuring protein and
deoxyribonucleic acid (DNA) synthesis by radiolabeling procedures, and a
neutral red cell–viability assay. A minimum of 24 to 48 hours is
required to measure the effects of the trichothecene mycotoxins on cell
viability.
These mycotoxins inhibit protein synthesis in a variety of
eukaryotic cells.46–48 Similar sensitivity to T-2 toxin was
observed in established cell lines and primary cell cultures.46,48
Inhibition of protein synthesis is observed 5 minutes after exposure of
Vero cells to T-2 toxin, with a maximal response noted by 60 minutes
after the exposure.46 Researchers47 have concluded
that the trichothecene mycotoxins act by inhibiting either the
initiation or the elongation process of translation, by interfering with
peptidyl transferase activity.
Substantial inhibition of ribonucleic acid (RNA) synthesis (86%
inhibition) by trichothecene mycotoxin was observed in human (HeLa)
cells,47 but T-2 toxin had minor effects (15% inhibition) on
RNA synthesis in Vero cells.46 The trichothecene mycotoxin–
related inhibition of RNA synthesis is probably a secondary effect of
the inhibition of protein synthesis. Scheduled DNA synthesis is strongly
inhibited in various types of cells that are exposed to trichothecene
mycotoxins. In mice or rats treated with trichothecene mycotoxins, DNA
synthesis in all tissues studied was suppressed, although to a lesser
degree than protein synthesis.49 The pattern by which DNA
synthesis is inhibited by the trichothecene mycotoxins is consistent
with the primary effect of these toxins on protein synthesis. In
appropriate cell models, for the most part, trichothecene mycotoxins
demonstrate neither mutagenic activity nor the capacity to damage DNA.50
Studies with radiolabeled trichothecene mycotoxins suggest that the
toxin interaction with cells is best viewed as (1) a free, bidirectional
movement of these low-molecular-weight chemicals across the plasma
membrane; and (2) specific, high-affinity binding to ribosomes.51
Thus, further evidence indicates that the primary toxic effects of the
trichothecene mycotoxins is caused by their properties as potent
inhibitors of protein synthesis.
Since the trichothecene mycotoxins are amphophilic molecules, an
investigation52 that focused on various kinds of interaction
with cellular membranes concluded that T-2 exerts multiple effects on
the cell membrane. Lipid peroxidation is increased in liver, spleen,
kidney, and thymus; and bone marrow when rats are treated with a single,
oral dose of T-2 toxin.53 These observations led to the
suggestion that the trichothecene mycotoxins might induce some
alterations in membrane structure, which consequently stimulates lipid
peroxidation. Once trichothecene mycotoxins cross the plasma membrane
barrier, they enter the cell, where they can interact with a number of
targets, including ribosomes 47 and mitochondria.54
These toxins inhibit electron transport activity, as the inhibition
of succinic dehydrogenase activity and mitochondrial protein synthesis
implies. Toxin-stimulated alteration in mitochondrial membranes
contributes to the effects on cellular energetics and cellular
cytotoxicity. Although initial investigations on the mechanism of action
of the trichothecene mycotoxins suggested that the inhibition of protein
synthesis as the principal mechanism of action, the above observations
indicate that the effects of these toxins are much more diverse.
|
|
Metabolism
Compared with some of the other mycotoxins such as aflatoxin, the
trichothecenes do not appear to require metabolic activation to exert
their biological activity.50 After direct dermal application
or oral ingestion, the trichothecene mycotoxins can cause rapid
irritation to the skin or intestinal mucosa. In cell-free systems or
single cells in culture, these mycotoxins cause a rapid inhibition of
protein synthesis and polyribosomal disaggregation.35,47,50
Thus, we can postulate that the trichothecene mycotoxins have molecular
capability of direct reaction with cellular components. Despite this
direct effect, it is possible to measure the toxicokinetics and the
metabolism of the trichothecene mycotoxins.
The lipophilic nature of these toxins suggests that they are easily
absorbed through skin, gut, and pulmonary mucosa. Absorption of a
single, oral dose of T-2 toxin is rapid, with concentration of labeled
toxin peaking in the blood within 1 hour.55 This indicates
that the trichothecene mycotoxins rapidly pass through the intestinal
mucosa. The inhaled median lethal dose of T-2 toxin is equal to 29
or less than28,30 the systemic dose. Mice, rats, and guinea
pigs die rapidly (within 1–12 h) after exposure to high concentrations
of aerosolized mycotoxin, with no apparent lung lesions or pulmonary
edema.28–30 This finding is in contrast to the effect of an
oral dose of T-2 toxin, which causes direct damage to the intestinal
mucosa.55
From these data, we can conclude that the trichothecene mycotoxins
very rapidly cross the pulmonary and intestinal mucosa and enter the
systemic circulation to induce the toxin-related toxicoses. In contrast,
trichothecene mycotoxins are only slowly absorbed through skin,
especially when applied as a dust or powder.56 Systemic
toxicity and lethality can be produced by dermal exposure to higher
concentrations of T-2 toxin, however, especially if the mycotoxin is
dissolved in a penetrant such as DMSO.6
Various cell culture lines and ruminal bacteria metabolize T-2
toxin by deacylation of specific deepoxidylation (ie, removal of the
oxygen from the epoxide ring at the C-12, 13 position to yield a
carbon–carbon double bond) and oxidization of the C-3 ' and C-4 '
positions on the isovaleryl side chains of T-2 toxin and HT-2 toxin, a
metabolite (Figure 34-2).57–59 A number of different cell
types contain the metabolic processes necessary to metabolize
trichothecene mycotoxins.
Pharmacokinetic studies60,61 have demonstrated T-2 toxin
in the plasma of animals that were administered this mycotoxin both
intravascularly and by aerosol. As plasma concentrations of the parent
trichothecene mycotoxin decrease, the deacylated and hydroxylated
metabolites and their glucuronide conjugates rapidly appear and
disappear from circulation. From these various observations, we can
conclude that the pharmacokinetics of the trichothecene mycotoxins are
functions of the rate of absorption into the general circulation,
metabolism, tissue distribution, and excretion.
Tissue-distribution studies55 suggest that the liver is
the major organ for metabolism of the trichothecene mycotoxins. The bile
and the gastrointestinal tract contained large amounts of radioactivity
after intravascular, intramuscular, oral, or dermal administration of
radiolabeled T-2 toxin. Although the liver is the major organ for the
metabolism of the trichothecene mycotoxins, other tissues such as the
intestine are capable of metabolic alteration of these toxins. After an
intravenous dose of T-2 toxin, 95% of the total radioactivity was
excreted in the urine and feces, in a ratio of 3 to 1.61 The
majority of the excreted products were either metabolites or glucuronide
conjugates of the metabolites.
Regardless of the route of administration or the species of animal
tested, the trichothecene mycotoxins were rapidly metabolized and
excreted in urine and feces. The route of exposure to the toxins and the
species can, however, influence the pattern of metabolites that are
excreted in the urine. The deacetylated and hydroxylated metabolites
appear to be present in most of the species that have been evaluated to
date.
A microsomal, nonspecific carboxylesterase [EC 3.1.1.1] from liver
selectively hydrolyses the C-4 acetyl group of T-2 toxin to yield HT-2
toxin.62 In addition to hepatic microsomes, the trichothecene-specific
carboxylesterase activity has been detected in brain, kidney, spleen,
intestine, white blood cells, and erythrocytes. These findings emphasize
the importance of carboxylesterase in detoxifying the trichothecene
mycotoxins. A hepatic cytochrome, P-450, is responsible for catalyzing
the hydroxylation of the C-3 ' and C-4 ' positions on the isovaleryl
side chain of the T-2 and HT-2 toxins.59 When oxygen is
removed from the epoxide group of a trichothecene mycotoxin to yield the
carbon–carbon bond, deepoxy metabolites are formed. The deepoxy
metabolites are essentially nontoxic.58 This latter
observation indicates that epoxide reduction is a single-step
detoxification reaction for trichothecene mycotoxins.
Four hours after swine received intravenous tritium-labeled T-2
toxin, glucuronide conjugates represented 63% of the metabolic residues
in urine, and 77% in bile.63 The formation of glucuronide
conjugates generally results in the elimination of toxicological
activity of xenobiotics, which in certain species could represent a
major route of detoxification of trichothecene mycotoxins. In summary,
then, very little of the parent trichothecene mycotoxin is excreted
intact. Rather, elimination by detoxification of the toxin is the result
of extensive and rapid biotransformation.
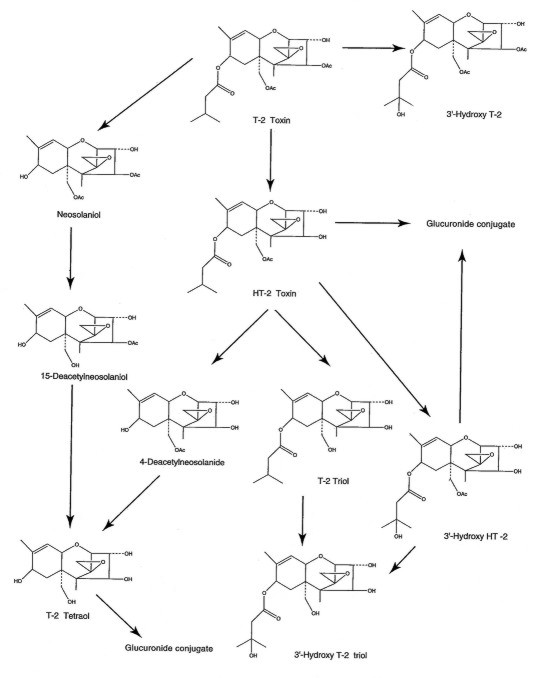 |
|
Fig. 34-2. Metabolic pathway of T-2 toxin both in vitro and
in vivo. |
|
|
The degree of illness in an individual exposed to trichothecene
mycotoxins could be affected by a number of factors, including the
nutritional status of the host, liver damage, intestinal infections,
route of toxin administration, and stress.
The pathological effects and clinical signs for many toxic
materials can vary with the route and type (acute, single dose vs
chronic, subacute doses) of exposure. For the trichothecene mycotoxins,
however, a number of the toxic responses are similar, regardless of the
route of exposure. As we discussed earlier in this chapter, once they
enter the systemic circulation, trichothecene mycotoxins affect rapidly
proliferating tissue regardless of the route of exposure.
In contrast, the symptoms and clinical signs of trichothecene
intoxication can vary depending on whether the exposure is acute or
chronic. Acute exposure to trichothecene mycotoxins used as biological
warfare agents is the major concern for military medicine, but for
continuity and historical implications, chronic intoxication will also
be addressed in this chapter.
Acute Effects
Acute oral, parenteral, dermal, or aerosol exposures to
trichothecene mycotoxins produce gastric and intestinal lesions.
Hematopoietic and immunosuppressive effects are radiomimetic. Central
nervous system toxicity causes anorexia, lassitude, and nausea;
suppression of reproductive organ function; and acute vascular effects
leading to hypotension and shock. While a number of toxic effects are
common to different routes of exposure, route-specific effects have been
observed in animal models. Examples of local, route-specific effects
include the following:
- dermal exposure:
local cutaneous necrosis and inflammation6;
- oral exposure:
lesions to the upper gastrointestinal tract64; and
- ocular exposure:
corneal injury.6
In Southeast Asia
during the 1970s, symptoms began within minutes after an exploding
munition (air-to-surface rocket, aerial bomb, cylinder) caused a yellow,
oily, droplet mist to fall on individuals within 100 m of the explosion
site. The falling droplet rain was inhaled, swallowed, and collected on
skin and clothing; contaminated the terrain and food and water supply;
and caused humans and animals to become acutely ill and to die after a
variable period.7 Massive cutaneous contact was prevalent
when the sources of exposure were sprays or coarse mists that were used
deliberately to contaminate humans and the environment. Although the
suspected trichothecene mycotoxin attacks in Southeast Asia would have
involved multiple routes of exposure, we can postulate that the skin
would have been the major site for deposition of a aerosol spray or
coarse mist.
Early symptoms and signs included severe nausea, vomiting, burning
superficial skin discomfort, lethargy, weakness, dizziness, and loss of
coordination. Within minutes to hours, diarrhea—at first watery brown
and later grossly bloody—began. During the first 3 to 12 hours, dyspnea,
coughing, sore mouth, bleeding gums, epistaxis, hematemesis, abdominal
pain, and central chest pain could occur. The exposed cutaneous areas
could become red, tender, swollen, painful, or pruritic, in any
combination. Small or large vesicles and bullae might form; and
petechiae, ecchymoses, and black, leathery areas of necrosis might
appear. After death, the necrotic areas might slough easily when the
corpse was moved.
Marked anorexia and dehydration were frequent. Dying patients
became hypothermic and hypotensive, and developed tachycardia. A bloody
ooze from the nares and mouth and an associated hematochezia occurred in
severely poisoned individuals. Death could occur within minutes, hours,
or days, and was often preceded by tremors, seizures, and coma, in any
combination.
The most common symptoms in both Southeast Asia and Afghanistan
included vomiting (71%); diarrhea (53%); skin irritation, burning, and
itching (44%); rash or blisters (33%); bleeding (52%); and dyspnea
(48%).7,15,27 All of the symptoms listed could be attributed
to trichothecene mycotoxin toxicity.
Dermal Exposure
Similar cutaneous irritations have been observed in numerous
accidental and experimental settings:
- Individuals who were
exposed to hay or hay dust contaminated with trichothecene-producing
molds developed severe cutaneous irritations.38
- In working up large
batches of fungal cultures from trichothecene-producing organisms,
laboratory personnel suffered facial inflammation followed by
desquamation of the skin and considerable local irritation.65
- When trichothecene
mycotoxins of relatively low toxicity (crotocin and trichotecin) were
applied to the volar surface of human forearm or to the human head,
reddening and irritation occurred within a few hours of exposure, and
was followed by inflammation or scrabbling that healed in 1 to 2
weeks.66
- The hands of two
laboratory workers were exposed to crude ethyl acetate extracts
containing T-2 toxin (approximately 200 µg/ mL) when the extract
accidently got inside their plastic gloves.66 Even though
the workers thoroughly washed their hands with a mild detergent within
2 minutes after contact, they experienced severe cutaneous
irritations.
a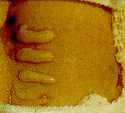  b b |
c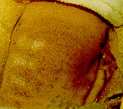 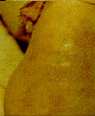 d d |
| Fig. 34-3.
Skin lesions on the back of a hairless guinea pig at (a) 1,
(b) 2, (c) 7, and (d) 14 days after application
of (bottom to top) 25, 50, 100, or 200 ng of T-2 toxin in 2 µL of
methanol. |
These observations
provide evidence that when human skin is exposed in vivo to small
amounts of trichothecene mycotoxins, severe cutaneous irritations
develop and can last 1 to 2 weeks after acute exposure.
A number of animal models have been used to assess local and
systemic toxicity and lethality from skin exposure to trichothecenes.6
In a dermal study that used a mouse model, necrosis in the skin was
present by 6 hours after dermal application of T-2 toxin, with
inflammation observed by 12 hours. The hairless guinea pig is an
excellent model to illustrate the local skin lesions produced by a
dermal application of T-2 toxin (Figure 34-3). The lesions are easily
identified by 24 hours after the exposure, with maximal response at 48
hours. Some small lesions are still present 14 days after exposure to
the toxin. From this experimental evidence, we can postulate that dermal
exposure to trichothecene mycotoxins played a major role in the clinical
illnesses that were seen following the yellow rain attacks.
Ocular Exposure
Victims of yellow rain attacks frequently reported tearing, eye
pain, conjunctivitis, burning sensations about the eyes, and blurred
vision for up to 1 week.7,16 A Canadian Forces medical team
interviewed Khmer Rouge causalities after a chemical/toxin attack at
Tuol Chrey, Kampuchea.27 Soldiers located 100 to 300 m from
the artillery impact had onset of symptoms 2 to 5 minutes after
exposure; these, likewise, included tearing, burning sensations, and
blurred vision that lasted from 8 to 14 days. Analysis of autopsy
samples from one of the casualties identified T-2, HT-2, and
diacetoxy-scripenol (DAS, also called anguidine) in his tissues. When
the culture filtrates containing trichothecenes were instilled into the
conjunctival sacs of rabbits, reddening and edema of the conjunctive
membrane were observed within 1 or 2 days. Later, the cornea became
opaque and developed scars that persisted for as long as 5 months.67
From these reports, we conclude that trichothecene mycotoxins can
cause severe eye injury that can lead to a marked impairment of vision.
This could be a severe incapacitating problem for unprotected military
personnel. No systemic toxicity has been documented from the
instillation of trichothecene mycotoxins into the eye of experimental
animals, however.
Respiratory Exposure
Victims of yellow rain reported a variety of upper respiratory
signs and symptoms.7,27 The major subdivisions of the
respiratory tract that were affected include the nose (itching, pain,
rhinorrhea, and epistaxis); the throat (sore/pain, aphonia, and voice
change); and the tracheobronchial tree (cough, hemoptysis, dyspnea, and
deep chest pain or pressure or both). Agricultural workers who were
exposed to hay or hay dust contaminated with trichothecene mycotoxins
developed similar signs and symptoms of upper respiratory injury. The
descriptions of the yellow rain attacks in Southeast Asia (ie, the
droplets, heavy mist, vapor), suggest that the aerosols were larger than
1 to 4 µm—the particle size required for deposition in the alveoli.
Thus, respiratory tract exposure from the larger-particle aerosols would
involve mycotoxin deposition in the upper respiratory and
tracheobronchial region, followed by secondary gastrointestinal tract
exposure after clearance from the lungs.
We can postulate that multiple routes of exposure (topical, upper
respiratory, and secondary enteral) to trichothecene mycotoxins occurred
in victims of the yellow rain attacks. The symptoms of vomiting,
diarrhea, melena, abdominal pain, and acute gastroenteritis with
hematemesis7 could be related to ingestion of toxin that was
deposited in the upper respiratory tract and tracheobronchial region.
Autopsies in the field of victims who died 24 to 48 hours after a yellow
rain attack disclosed severe gastroenteritis with bleeding in the lower
esophagus, stomach, and duodenum.27 In humans, many of the
acute enteral effects (from either yellow rain or contaminated hay and
dust particles) of the trichothecene mycotoxins are probably the result
of secondary ingestion of toxins that originally were deposited in the
respiratory tract by large-particle aerosol.
Chronic Toxicity
Chronic exposure to subacute doses of trichothecene mycotoxins is
not thought to be an effect of biological warfare. This type of
exposure, however, was responsible for ATA toxicosis in humans and
mycotoxicosis in domestic animals. In addition, chronic toxicity has
been iatrogenically induced when repeated subacute doses of a
trichothecene mycotoxin were administrated intravenously to cancer
patients as a chemotherapy for colon adenocarcinoma.
Alimentary Toxic Aleukia Toxicosis
The clinical course of ATA is divided into four stages. The
first stage develops immediately or several days after consumption
of grain products that are contaminated with trichothecene mycotoxins.
Inflammation of the gastric and intestinal mucosa causes vomiting,
diarrhea, and abdominal pain. In most cases, excessive salivation,
headache, dizziness, weakness, fatigue, and tachycardia accompany this
stage, and fever and sweating may also be present.36
The disease progress to the second stage—the leukopenic or
latent stage—which is characterized by leukopenia, granulopenia, and
progressive lymphocytosis. When the ingestion of the toxin-contaminated
food is not interrupted or if large doses are consumed, the next stage
develops.36
The third stage is characterized by the appearance of a
bright red, or dark cherry-red, petechial rash on the skin of the chest
and other areas of the body. At first, the petechiae are localized in
small areas, but they then spread and become more numerous.
In the most severe cases, intensive ulceration and gangrenous
processes develop in the larynx, leading to aphonia and death by
strangulation. At the same time, affected individuals have severe
hemorrhagic diathesis of the nasal, oral, gastric, and intestinal
mucosa.36
As the necrotic lesions heal and the body temperature falls, the
fourth stage—the recovery stage—begins. During this period, exposed
patients are susceptible to various secondary infections, including
pneumonia. Convalescence is prolonged and can last for several weeks.
Usually, 2 months or more are required for the blood-forming capacity of
the bone marrow to return to normal.36
Cancer Chemotherapy
The inhibitory effect of trichothecene mycotoxins on rapidly
dividing cells was the basis for their evaluation as antitumor
chemotherapy drugs during the late 1970s and early 1980s.68
Phase I and phase II clinical evaluations of DAS (anguidine) in patients
with cancer disclosed significant toxicity with intravenous doses 3.0
mg/m 2 (0.077 mg/kg) daily for 5 days, particularly in
patients with hepatic metastases. The signs and symptoms included
nausea, vomiting, diarrhea, burning erythema, confusion, ataxia, chills,
fever, hypotension, and hair loss.69,70 Antitumor activity of
the trichothecenes was minimal or absent in the patients treated with
DAS. Because of the marked toxicity of the drug, the life-threatening
hypotensive effects, and the poor tolerance by patients, the evaluation
of trichothecenes as chemotherapeutic drugs was discontinued. |
|
Battlefield Diagnosis
In the absence of a biological detector or a particular
characteristic of the aerosol (such as color or odor), diagnosis of an
attack with trichothecene would depend on clinical observations of
casualties and identification of the toxins in biological or
environmental samples. This would involve a combined effort between the
medical and chemical units in the field. The early signs and symptoms of
an aerosol exposure to trichothecene mycotoxins would depend on particle
size and toxin concentration. For a large-particle aerosol (particles >
10 µm, found in mist, fog, and dust; similar to that used in Southeast
Asia), the signs and symptoms would include rhinorrhea, sore throat,
blurred vision, vomiting, diarrhea, skin irritation (burning and
itching), and dyspnea. Early (0–8 h) signs and symptoms from a
deep-respiratory aerosol exposure (from aerosol particles in the 1- to
4-µm range) have not been fully evaluated but could include vomiting,
diarrhea, skin irritation, and blurred vision.
Later signs and symptoms (8–24 h) would probably be similar (except
for the degree of skin rash and blisters) for both large-particle and
deep-respiratory aerosol exposure to trichothecene mycotoxins. They
could include continued nausea and vomiting, diarrhea, burning erythema,
skin rash and blisters, confusion, ataxia, chills, fever, hypotension,
and bleeding.
Nonspecific changes in serum chemistry and hematology occurred in
monkeys exposed to an acute dose of T-2 toxin. Alterations in serum
chemistry included elevations in serum creatinine, serum enzymes
(especially creatine kinase), potassium, phosphorous, and serum amino
acids; and, due to decreased coagulation factors, elevations in
prothrombin time and partial thromboplastin time. An initial rise in the
absolute number of neutrophils and lymphocytes may occur within hours,
followed by a decrease in lymphocyte counts by 48 hours. Survival beyond
several days may be associated with a fall in all blood cellular
elements.6 Although it is likely that these acute changes
will also be seen in humans, careful clinical observations of human
victims of acute trichothecene mycotoxicosis have not been reported to
date. In patients with chronic toxicity (ie, ALA) resulting from
repeated ingestion of contaminated bread, pancytopenia is an important
part of the clinical picture.36
In the yellow rain attacks in Southeast Asia, diagnosis of the
causative agent was difficult and involved ruling out the presence of
conventional chemical warfare agents. Contamination of the environment
and clothing by nerve and blistering agents would be absent, and these
were, in fact, not detectable in such samples from Southeast Asia.
Sarin, soman, or other nerve agents could be missed unless thickened
soman or VX was used.
The following events should suggest to medical officers that a
biological warfare attack with trichothecene mycotoxins has occurred:
- clinical findings
that match the symptoms listed above;
- high attack and
fatality rates;
- all types of dead
animals; and
- onset of symptoms
after a yellow rain or red, green, or white smoke or vapor attack.
At present, we do
not have a fieldable identification kit for any of the trichothecene
mycotoxins. Several commercial immunoassay kits are marketed for the
detection of trichothecene mycotoxins (T-2 toxin, deoxynivalenol, and
their metabolites) in grain extracts or culture filtrates of Fusarium
species.71,72 These kits have not been evaluated against
biomedical samples that contain typical concentrations of the
mycotoxins, however. Screening tests for presumptive identification of
trichothecene mycotoxins in the biomedical samples would probably
involve bioassays, thin-layer chromatography, or immunological assays,
in any combination. At a national laboratory, confirmatory methodology
would involve the use of various combinations of gas chromatography,
high-performance liquid chromatography, mass spectrometry, and nuclear
magnetic resonance spectrometry.
In areas that have experienced a yellow rain attack, environmental
assays have been in the range of 1 to 150 parts per million (ppm) and
blood samples in the range of 1 to 296 parts per billion (ppb).8–10,16,22
In the laboratory, at 10 and 50 minutes after an intramuscular exposure
to 0.4 mg/kg of T-2 toxin in the dog, plasma concentrations of T-2 toxin
were 150 and 25 ppb, and for HT-2 toxin were 50 and 75 ppb,
respectively.60 Thus, any screening procedure for
trichothecene mycotoxins in biomedical samples must have detection
limits of 1 to 100 ppb. Most of the analytical procedures require
extraction and cleanup treatment to remove interfering substances.73
Screening tests for the trichothecene mycotoxins are generally
simple and rapid but, with the exception of the immunochemical methods,
are nonspecific. A number of bioassay systems have been used for the
identification of trichothecene mycotoxins.73 Although most
of these systems are very simple, they are not specific, their
sensitivity is generally relatively low compared to other methods, and
they require that the laboratory maintain vertebrates, invertebrates,
plants, or cell cultures. Thin-layer chromatography (TLC) is one of the
simplest and earliest analytical methods developed for mycotoxin
analysis. Detection limits for trichothecene mycotoxins by TLC is 0.2 to
5 ppm (0.2 to 5 µg/ mL). Therefore, extracts from biomedical samples
would have to be concentrated 10- to 1,000-fold to screen for
trichothecene mycotoxins.
To overcome the difficulties encountered with the bioassays and TLC
methods, immunoassays using specific polyclonal and monoclonal
antibodies have been developed for most of the major trichothecene
mycotoxins and their metabolites.73 These antibodies have
been used to produce simple, sensitive, and specific radioimmunoassays (RIAs)
and enzyme-linked immunosorbent assays (ELISAs) for the mycotoxins. In
the presence of the sample matrix, the lower detection limits for
identification of trichothecene mycotoxins by RIA is about 2 to 5 ppb73
and by ELISA, 1 ppb.74 We conclude that immunoassays are
useful tools for screening biomedical samples for evidence of a
biological warfare attack with trichothecene mycotoxins.
Confirmatory Procedures
Gas-liquid chromatography (GLC) is one of the most commonly used
methods for the identification of the trichothecene mycotoxins in both
agricultural products and biomedical samples.75 Before GLC
analysis, the polar groups in mycotoxin molecules must first be
converted to their esters or ethers. Extensive treatment to clean up the
sample is required before derivatization and subsequent analysis can be
performed. By the most sensitive procedures, the detection limit for
trichothecene mycotoxins is 10 ppb. If the analysis is on a sample that
contains an unknown toxic material, such as those from the yellow rain
attacks, then the GLC method can only provide presumptive evidence of a
trichothecene mycotoxin exposure. Confirmation will require the
identification with more definitive physicochemical procedures.
Mass spectrometry (MS) is the physicochemical method of choice for
characterizing, identifying, and confirming the presence of
trichothecene mycotoxins.76,77 Picogram quantities of
trichothecene mycotoxins are readily detectable by MS methods. In some
cases, extensive cleanup steps are unnecessary.
The combination of GLC and MS techniques (GLC–MS) has proven to be
a more-specific method for identifying mycotoxins than is GLC alone.76,77
As a result, the GLC–MS method has become the standard for identifying
trichothecene mycotoxins in agricultural products as well as in
biomedical samples. As little as 1 ppb of T-2 toxin can be identified
without extensive cleanup.76 One major drawback of this
methodology is the time-consuming derivatization step that trichothecene
mycotoxin identification by GLC–MS requires. A high-performance liquid
chromatography–mass spectrometry (HPLC–MS) procedure was described in
1991 and provides a specific and reliable method for the identification
of trichothecene mycotoxins without derivatization.78 The
HPLC–MS procedure achieves sensitivity at the 0.1-ppb level. This
technology will require further evaluation and development, but it
appears to be a promising approach for the rapid confirmation of
trichothecene mycotoxins in a biomedical sample. |
|
Individual and Unit
The immediate use of protective clothing and mask at the first sign of a
yellow rain–like attack should protect an individual from the lethal
effects of this mycotoxin. The mask can be applied in less than 9
seconds and can be worn at first sighting of an incoming rocket or enemy
aircraft. Contaminated battle dress uniforms (BDUs) should be removed
before protective clothing is donned. Since the area covered with agent
is likely to be small, another helpful tactic is to leave the area after
taking samples to document the attack. Vulnerability is increased by
lack of protective clothing, mask, or training (as was demonstrated in
Laos) or by a surprise biological warfare attack (such as a night or an
undetected attack). A lightweight face mask, outfitted with filters that
block the penetration of aerosol particles 3 to 4 µm or larger, should
provide respiratory protection against yellow rain. Only 1% or 2% of
aerosolized T-2 toxin penetrated nuclear, biological, chemical
protective covers (NBC–PC).79 Regular BDUs would offer some
protection, but the degree would be functions of the age and condition
of the fabric, and the type of environmental conditions.
Two topical skin protectants (TPS1 and TSP2) are in advanced
development for protection against chemical warfare agents. When applied
to the skin of rabbits 60 minutes before exposure to 50 µg of T-2 toxin,
both topical skin protectants completely protected the rabbits from the
dermal irritating effects of this mycotoxin for at least 6 hours.80
As soon as individuals or units suspect that they have been exposed
to a mycotoxin attack, they should remove their BDUs, wash their
contaminated skin with soap and water, and then rinse with water.
Washing the contaminated area of the skin within 4 to 6 hours after
exposure to T-2 toxin removed 80% to 98% of the toxin and prevented der-mal
lesions and death in experimental animals.25 Contaminated
BDUs as well as wash waste from personnel decontamination should be
exposed to household bleach (5% sodium hypochlorite) for 6 hours or more
to inactivate any residue mycotoxin.
Two skin decontamination kits, the M258A1 and the M238A1, have been
designed for the removal and detoxification of chemical warfare agents.
The M258A1 kit is the currently fielded standard. When evaluated against
trichothecene mycotoxins, however, the M238A1 kit effectively removed
T-2 toxin from the skin of rats but did not detoxify this biological
warfare agent.81 Several of the components of the M258A1 kit
are themselves highly toxic, caustic compounds that caused dermal
irritation and lethality in rats and rabbits.82
A second-generation skin decontamination kit, the XM291, has been
developed, and contains an XE-555 resin material as the active
component. This skin decontamination kit is efficacious against most
chemical warfare agents and presents no serious human factor or human
safety problems. The XE-556 resin, a similar but different formulation,
was effective in the physical removal of T-2 toxin from the skin of
rabbits and guinea pigs.83 The foregoing observations suggest
that the skin decontamination kits that were designed specifically for
removal and detoxification of chemical warfare agents could also afford
a significant degree of protection through the physical removal of
mycotoxins from the skin of exposed individuals.
Specific or Supportive Therapy
No specific therapy for trichothecene-induced mycotoxicosis is
known or is presently under experimental evaluation. Several therapeutic
approaches have been evaluated in animal models. It is perhaps
significant, however, that although experimental procedures for
treatment of systemic exposure have been successful in reducing
mortality in animal models, they have not been tested in primates. Thus,
these treatments are not available for field use for humans exposed to
trichothecene mycotoxins.
Individuals exposed to a yellow rain–like attack should be treated
with standardized clinical toxicology and emergency medicine practices
for ingestion of toxic compounds. After an aerosol exposure to a yellow
rain–like attack, mycotoxins will be trapped in the nose, throat, and
upper respiratory tract. The particles will be returned by ciliary
action to be swallowed, resulting in a significant oral exposure.
Superactive charcoal has a very high maximal binding capacity (0.48 mg
of T-2 toxin per 1 mg of charcoal), and treatment either immediately or
1 hour after oral or parenteral exposure to T-2 toxin significantly
improves the survival of mice.84 Superactivated charcoal with
magnesium sulfate is stocked in the chemical and biological warfare kits
of U.S. Army field hospitals.
Symptomatic measures for the treatment of exposure to trichothecene
mycotoxins are modeled after the care of casualties of mustard
poisoning.85 Irrigation of the eyes with large volumes of
isotonic saline may assist in the mechanical removal of trichothecene
mycotoxins, but would have limited useful therapeutic effects. After the
skin has been decontaminated, some erythema may appear, accompanied by
burning and itching. Most casualties whose skin has been treated with
soap and water within 12 hours of exposure will have mild dermal
effects; these should be relieved by calamine and other lotion or cream,
such as 0.25% camphor and methanol.
Limited data are available on the respiratory effects of inhaled
trichothecene mycotoxins, although acute pulmonary edema is one of the
serious, often lethal consequences of a yellow rain attack.16,27
One of the major symptoms following the yellow rain attacks was an upper
respiratory irritation (sore throat, hoarseness, nonproductive cough),7,16,27
which can be relieved by steam inhalation, codeine, or another substance
to suppress the cough, and other simple measures.85 A
casualty who develops severe respiratory symptoms should be under the
care of a physician skilled in respiratory care.
The early use of high doses of systemic glucocorticosteriods
increases survival time by decreasing the primary injury and the
shocklike state that follows exposure to trichothecene mycotoxins.86
A selective platelet activating factor antagonist, BN 52021, can prolong
the survival of rats exposed to a lethal intravenous dose of T-2 toxin.87
This finding suggests that platelet activating factor is an important
mediator of T-2 toxicosis. Dosing before and after the exposure with
diphenhydramine (an antihistaminic agent) or naloxone (an opioid
antagonist) prolonged the survival times of mice exposed subcutaneously
or topically with lethal doses of T-2 toxin.88
We can postulate that a number of bioregulators are the mediators
of the shocklike state of trichothecene mycotoxicosis.
Methylthiazolidine-4-carboxylate increased hepatic glutathione content
and enhanced the survival of mice after an acute intraperitoneal
exposure to T-2 toxin.89 The protective effects of this drug
may be the result of increased detoxification and excretion of the
glucuronide conjugate of T-2 toxin. A general therapeutic protocol that
included combinations of metoclopramide, activated charcoal, magnesium
sulfate, dexamethasone, sodium phosphate (which had very little effect),
sodium bicarbonate, and normal saline as the therapeutic agents was
evaluated in swine given an intravenous LD50 dose of T-2
toxin.90 All treatment groups showed improved survival times
when compared with the nontreated T-2 controls.
Prophylaxis
The mycotoxins are low-molecular-weight compounds that must be
conjugated to a carrier protein to produce an effective antigen.73
When T-2 toxin is conjugated to a protein, it develops relatively low
antibody titers and is still a marked skin irritant.91 This
would preclude mycotoxins’ use as immunogens in the production of
protective immunity. To circumvent such problems, a deoxyverrucarol
(DOVE)–protein conjugate was used to immunize rabbits.92
Antibody titers to DOVE developed rapidly after immunization, but they
were highly specific for DOVE rather than a common trichothecene
backbone.92
Another approach was to develop antibody-based vaccines (anti-idiotype)
against T-2 toxin. Protective monoclonal antitoxin antibodies were first
generated and then used to induce specific monoclonal anti-idiotype
antibodies. When mice were immunized with specific monoclonal anti-idiotype
antibodies, they developed neutralizing antibodies and were protected
against challenge with a lethal dose of T-2 toxin.93
Thus, it would be feasible to develop a despeciated monoclonal
anti-idiotype antibody that could be a vaccine candidate against T-2
toxin. Several monoclonal antibodies against T-2 toxin will protect
against the T-2–induced cytotoxicity in various cell lines.94,95
When a monoclonal antibody against T-2 toxin (15H6) was given to rats
(250 mg/kg) 30 minutes before or 15 minutes after a lethal dose of
mycotoxin, it conferred 100% survival.94 Thus, monoclonal
antibodies do have some prophylactic and therapeutic value against T-2
toxicosis, but very large quantities are required for protection.
Prophylactic induction of enzymes involved in the conjugation of
xenobiotics reduced or prevented the acute toxic effects of T-2 toxin in
the rat, while inhibition of these enzymes resulted in a higher toxicity
for this trichothecene.96 Pretreatment with flavonoids,97
ascorbic acid,98 vitamin E,99 selenium,100
or chemoprotective compounds such as emetine101 that block
trichothecene–cell association all reduce acute toxicity of these
mycotoxins. However, none of these chemoprotective treatments have
undergone extensive efficacy studies to evaluate their ability to
protect against an aerosol or dermal exposure to trichothecene
mycotoxins.
|
|
Trichothecene mycotoxins are noted for their marked stability under
different environmental conditions. On a weight-for-weight basis, they
are less toxic than other toxins such as ricin, botulinum, and
staphylococcal enterotoxin B, but trichothecene mycotoxins are proven
lethal agents in warfare. Symptoms include vomiting, pain, weakness,
dizziness, ataxia, anorexia, diarrhea, bleeding, skin redness,
blistering, and gangrene, as well as shock and rapid death. Sensitive
immunoassays and chemical procedures are available for the
identification of trichothecene mycotoxins in biological samples, but no
detection kits have been fielded.
Prevention of exposure is the only current defense, with a
protective mask and clothing worn when under attack. Previous successful
lethal attacks have always occurred against unprotected civilians and
soldiers. Skin decontamination with water and soap can be used
effectively up to 6 hours after exposure. Experimental treatments for
systemic toxicity are being investigated, but no therapy is available
for humans.
|
|



 b
b
 d
d