Indoor dampness and
mold problems are universal and thus are potentially of major public
health importance (1-11). Such problems have been surprisingly
common in countries with cold climates, such as Finland, Sweden, and
Norway (4,5,9,11). The major reasons for the high frequency of
such problems in cold climates may be insufficient maintenance of the
buildings and construction of tight buildings to conserve energy
accompanied by inadequate ventilation. Residential dampness problems
have been related to increased risk of asthma and asthma-related
symptoms in children (1,2,4,6,8,12-15) and in adults (7,9,13,15-20).
However, we did not identify any epidemiologic study of workplace indoor
dampness and mold problems and asthma, and only four studies have
evaluated potential effects of such problems on wheezing (5,10,21,22).
These studies were carried out in either daycare centers or offices.
Most of the studies among adults were cross-sectional or prevalent
case-control studies in design, and almost all of them based the
diagnosis of asthma or asthma-related symptoms on self-report in
questionnaires or interviews.
The objective of our
study was to assess the role of dampness problems and molds at work and
at home in the development of asthma in working-age population. We
recruited incident cases of asthma, the diagnosis being verified with
clinical examinations. We also evaluated some personal characteristics,
such as age, sex, and smoking, as potential indicators of sensitivity to
the adverse effects of dampness problems.
Study Design
This study was a
population-based incident case-control study. The source population
consisted of adults 21-63 years old living in the Pirkanmaa Hospital
district. This district is a geographically defined administrative area
in South Finland with a population of 440,913 inhabitants in 1997. Our
goal was to recruit all the new cases of asthma in the source population
during the study. We selected controls randomly from the source
population based on 1997 census data. The ethics committees of the
Finnish Institute of Occupational Health and the Tampere University
Hospital approved the study.
Definition and
Selection of Cases
We systematically
recruited all the new cases of asthma, first in the city of Tampere
beginning on 15 September 1997, and then in the whole Pirkanmaa Hospital
district from 10 March 1998 to 31 March 2000. We recruited patients at
all health care facilities diagnosing asthma, including the Department
of Pulmonary Medicine at the Tampere University Hospital, offices of the
private-practicing pulmonary physicians in the region, and public health
care centers. As an additional route of case selection, the National
Social Insurance Institution of Finland invited all patients to
participate whose reimbursement rights for asthma medication began
during the period 1 September 1997 through 1 May 1999 and who had not
yet participated.
We applied the
following diagnostic criteria for asthma: a) history of at least
one asthmalike symptom (prolonged cough, wheezing, attacks of or
exercise-induced dyspnea, or nocturnal cough or wheezing) and b)
demonstration of reversibility in airway obstruction in lung function
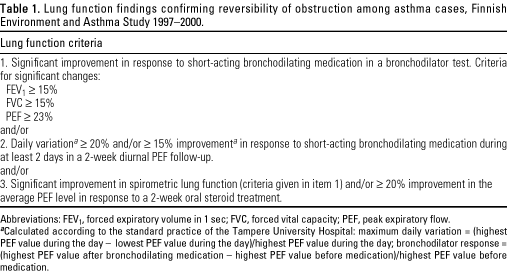
investigations. Table 1 presents lung function findings accepted to
demonstrate reversibility. These diagnostic procedures correspond to the
recommendations of the National Asthma Program in Finland (23).
We selected as cases
all the confirmed cases of asthma fulfilling the general eligibility
criteria. A total of 362 cases (response rate, 90%) participated through
the health care system, and 159 cases participated through the National
Social Insurance Institution (response rate, 78%), totaling 521 cases
overall.
Selection of
Controls
We randomly selected
the controls from the source population using the national population
registry, which has full coverage of the population. We applied the
general eligibility criteria for controls. After up to three invitation
letters and phone calls, 1,016 participated in the study (response rate,
67% of total invited population, or 80% of those who had a phone number
in the Pirkanmaa area). Previous or current asthma was reported by 76
(7.5%); six persons were older than 63 years, and two returned
incomplete questionnaires. After excluding these persons, our study
population included 932 controls.
Exposure Assessment
We based exposure
assessment on questionnaire information about water damage, damp stains
and other marks of structural dampness, visible mold, and mold odor,
both at home and indoors at work (4,5). For water damage, damp
stains, and visible mold, we asked for information about their
occurrence during the past year, 1-3 years before, or > 3 years
before. For mold odor, we asked the subject about occurrence during the
past year and to indicate if such odor appeared almost daily, 1-3
days a week, 1-3 days a month, < 1 day a month, or never.
Data Collection
At the Tampere
University Hospital, we recruited cases at their first visit for
suspected asthma, and we verified the diagnosis in clinical
examinations. At the other health care facilities, cases were recruited
immediately when their asthma diagnosis was verified. We applied the
same protocol for diagnosing asthma at all health care facilities. The
National Social Insurance Institution invited the cases 6 months to 2
years after their diagnosis was established. For these patients, we
confirmed the date and criteria of the asthma diagnosis from their
medical records to ensure that the diagnosis of asthmatics included in
our study fulfilled our criteria. For all cases, we verified from their
medical records that they did not have a previous asthma diagnosis.
Eligible subjects were invited to participate in the study, and informed
consent was asked by their physician or through a letter sent by the
National Social Insurance Institution. The cases answered the
questionnaire at the time of recruitment. Recruitment of controls took
place at regular intervals throughout the study period. Informed consent
was requested in the letter and returned in a prepaid envelope to the
research nurse of the study project.
Measurement Methods
Questionnaire.
The self-administered questionnaire, modified from the Helsinki
Office Environment Study questionnaire (24,25) to be used in a
general population, included six sections: 1) personal characteristics,
2) health information, 3) active smoking and environmental tobacco smoke
exposure, 4) occupation and work environment, 5) home environment, and
6) dietary questions.
Lung function
measurements. We applied the same lung function protocol to all
patients with suspected asthma. The only exception was patients
recruited through the National Social Insurance Institute, for whom we
obtained lung function data by abstracting from the medical records.
Baseline spirometry.
For all patients with suspected bronchial asthma, we recorded vital
capacity and flow-volume curves with a pneumotachygraph spirometer
connected to a computer and using a disposable flow transducer (Medikro
905; Medikro Ltd., Kuopio, Finland). We carried out the measurements
according to the standards of the American Thoracic Society (26).
We judged presence of obstruction using the reference values derived
from a Finnish population (27).
Bronchodilation
test. After baseline spirometry, all patients received 400 µg of
salbutamol (albuterol) with a spacer and performed spirometric
flow-volume curves after 10 min.
Peak expiratory flow
(PEF) follow-up. All patients performed PEF follow-up for at least 2
weeks with a mini Wright meter. We instructed subjects to carry out
measurements twice a day, in the morning and in the evening. During the
second week, subjects performed measurements before and 15 min after
short-acting bronchodilating medication. Subjects recorded all three
readings, and we used the highest value in the analyses.
Steroid treatment
response. We recommended that physicians give a 2-week oral steroid
treatment to those with a strong suspicion of asthma, if the other
diagnostic tests were negative. The patient was asked to perform 2 weeks
of PEF follow-up during this treatment, and spirometry was carried out
again at the end of this treatment period to judge the response.
Statistical Methods
We used exposure odds
ratio (OR) to quantify the relations between exposures and outcome, and
estimated adjusted OR in logistic regression analysis. We used the
following covariates to adjust for potential confounding: sex, age,
parental atopy or asthma, education (as an indicator of socioeconomic
status), personal smoking, dampness and mold problems in the home or at
work, exposure to environmental tobacco smoke, any history of pets in
the home, and self-reported occupational exposure to sensitizers, dusts
or fumes (except self-reported exposure to molds).
We studied the
independent predictive value of the four exposure indicators (water
damage, damp stains, visible mold, and mold odor) in the workplace (for
those working at least 50% of their workday indoors) and in the home by
including all the exposure indicators as well as covariates in the
model. We also elaborated the role of exposure time period by fitting
time-specific exposure variables. We combined occurrence of dampness and
mold problems during different time periods because we detected no
meaningful trends according to the time specificity of exposure (data
not shown). We combined any visible mold and/or mold odor in the
workplace to represent the main exposure parameter. These two exposure
indicators were closely related and had strong overlap, so including
them separately in the models was not meaningful. The reference category
consisted of those reporting no mold or dampness exposure. We also
analyzed the data after excluding patients recruited by the National
Social Insurance Institution.
We systematically
studied potential modification of the relation between main exposure
parameter and risk of asthma by comparing the adjusted ORs by sex, age
(20-29, 30-49, and 50-63 years), parental atopy or asthma (yes/no), and
smoking (never, former, current).
Finally, we quantified
the impact of exposure as an attributable fraction (28) or
etiologic fraction (29), providing the fraction of exposed cases
for whom the disease is attributable to the exposure (28). We
calculated the attributable fraction (AF)
AF = (OR - 1)/OR,
where OR is the
adjusted OR due to the exposure of interest, an unbiased estimate of
incidence ratio in a population-based case-control study (29). We
calculated the 95% confidence interval (CI) using the corresponding
interval of OR.
Characteristics and
Exposure of Cases and Controls
A larger proportion of
cases than controls were women, young, current smokers, and exposed to
environmental tobacco smoke and to pets; had lower education; and
reported a history of parental allergic diseases (Table 2).

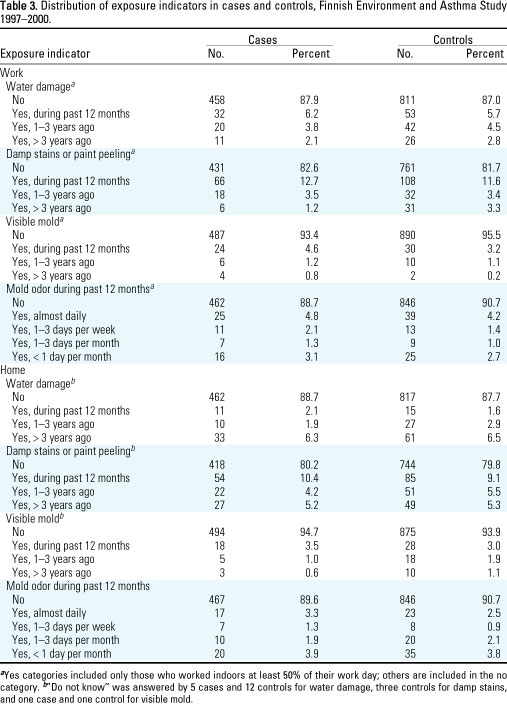
A larger percentage of
cases than controls reported presence of visible mold (6.6% vs. 4.5%)
and mold odor (11.3% vs. 9.3%) in the workplace (Table 3). The frequency
distributions of water damage and damp stains or paint peeling in the
workplace and of all the four exposure indicators in the home were
similar among cases and controls.
Indoor Dampness
Problems and Molds and the Risk of Asthma
The risk of asthma was
related to the presence of visible mold and/or mold odor in the
workplace, but not to water damage or damp stains alone, as shown in
Table 4. The adjusted OR for any exposure to visible mold or mold odor
was 1.54 (95% CI, 1.01-2.32). The risk of asthma was related to
none of the exposure indicators in the home. The results were
essentially similar in the analyses that excluded cases recruited
through the National Social Insurance Institution. We estimated the
fraction of asthma attributable to workplace mold exposure to be 35.1%
(95% CI, 1.0-56.9%) among the exposed.


The relation between
workplace mold exposure and the risk of asthma was slightly stronger in
women than in men (Table 5). The relation was strongest in the youngest
age group and stronger in current smokers than in former smokers or
never smokers. The relative risk was essentially similar in those with
and without parental atopy.
We found a
significantly increased risk of new asthma in adults in relation to the
presence of visible mold and/or mold odor in the workplace, whereas
water damage or damp stains alone were not associated with asthma. The
mechanisms by which indoor dampness problems could lead to an increased
risk of asthma are not well understood, and several potential causes
have been suggested: molds, bacteria, house dust mites, and enhanced
emission of chemicals from surface materials (9,15). Our results
emphasize the role of molds (and possibly bacteria) as an important
cause of asthma, rather than dampness per se. Potential mechanisms by
which indoor molds could induce asthma include immunoglobulin E-mediated
hypersensitivity reactions, toxic reactions caused by mycotoxins, and
nonspecific inflammatory reactions caused by irritative volatile organic
compounds produced by microbes or cell wall components, such as
1,3-ß-d-glucan and ergosterol (9,30-32). Different species of
molds may induce asthma by different mechanisms, or molds may induce
health effects by combined mechanisms (32).
The risk of asthma was
not associated with the presence of dampness or molds at home in this
study. We have no reason to believe that effects of similar exposures at
home and at work would be different. Rather, the difference in effect
estimates in our study are likely explained by more extensive mold
problems at work than at home. We did not quantify the extent of such
problems, but it is likely that in the workplace people do not notice
small dampness problems easily, because they change work areas often,
and thus more extensive mold growth may develop. In addition,
influencing the work environment is often more difficult. At home,
people tend to pay attention to water damage and repair it before more
advanced mold problems develop, because such damage reduces the value of
the property. In 1998 in the Pirkanmaa area, 67% of the population owned
the residences in which they lived. Other potential explanations for
these differences include, for example, ventilation systems of
workplaces favoring the spread of molds and their metabolites into
indoor air. The attributable fraction of asthma due to workplace mold
was surprisingly high: 35% among the exposed cases.
We found that women,
the young, and smokers are especially susceptible to the effects of
workplace molds. The mechanisms of such susceptibility are not known and
should be studied further. The young and women may have more extensive
exposures, because they are often in lower positions in the workplace
and therefore have less influence on their work environment. Also,
modification of immunologic or other inflammatory reactions may play a
role in sensitivity, at least in current smokers.
Validity Issues
We were able to recruit
a high proportion of new cases of asthma by a thorough recruitment
through the health care system (response rate, 90%) and with the help of
the National Social Insurance Institution providing us a route to reach
those asthmatics that we missed by our recruitment system (response
rate, 78%). The health insurance provided by the National Social
Insurance Institution covers the whole Finnish population, and its
medication files have practically a full coverage of asthmatics who
fulfill the diagnostic criteria required for reimbursement in Finland.
The response rate among the control population was also relatively high,
especially among those who had a phone number in the Pirkanmaa region
and were likely to really live in this area during our study period.
Thus, any major selection bias is unlikely in our study.
To reduce information
bias, we introduced the study to the participants as a study on
environmental factors and asthma in general (the Finnish Environment and
Asthma Study), with no special focus on mold and dampness problems. We
collected information on exposures in a similar way from cases and
controls. The physicians responsible for the diagnostic procedures of
asthma were unaware of the questionnaire responses of the study
subjects. Our finding of an increased risk of asthma in relation to
workplace exposure but not in relation to home exposure supports
unbiased reporting; it is unlikely that subjects would associate their
symptoms to a specific exposure in one environment but not in the other.
In the analyses excluding cases recruited by the National Social
Insurance Institution (i.e., some time after their diagnosis was made),
the OR related to workplace mold exposure remained increased (1.38) but
was slightly reduced. This indicates that some overreporting of
workplace exposure may have taken place, but this does not explain the
effect entirely. On the other hand, studies comparing self-reported
dampness with site visits have usually shown that subjects tend to
underestimate their exposures (7,9,11). Thus, self-report of
exposures may have led to some underestimation of the risks in our study
(e.g., the risks for home exposures). We defined asthma on the basis of
objective clinical findings to eliminate information bias concerning the
outcome.
We were able to adjust
for a number of potential confounders (see "Statistical Methods") in
logistic regression analysis to eliminate these factors as potential
explanations for our results. We adjusted for parental atopy to control
for genetic predisposition, but not for subjects' own atopy, because
this may be in the causal pathway for effects of indoor molds.
Synthesis with
Previous Knowledge
Earlier studies on
indoor dampness and mold problems in adults have been mainly
cross-sectional or prevalent case-control studies in design; therefore,
our results cannot be compared directly with them. One population-based
study from Sweden assessed adult-onset asthma based on questionnaire
reports of asthma (20). A significant OR of 2.2 was reported in
relation to visible mold at home, whereas visible dampness alone was not
significantly related to asthma. The Swedish study assessed the onset of
asthma retrospectively based on self-reported information about the year
of diagnosis. The subjects had to recall both the year of diagnosis and
exposures as far back as 14 years before, which makes the study
vulnerable to recall bias. The Swedish study did not adjust for or
estimate the risk related to workplace mold problems.
We identified no
earlier study that had assessed the relation between workplace dampness
and mold problems and the risk of asthma. A previous study in Finnish
daycare nurses reported the risk of wheezing related to workplace
exposure, while adjusting for home exposure (5). The OR was 1.66
when water damage was present and 1.28 when water damage and mold odor
both were present. These estimates are close to our OR of asthma among
women (1.67). In addition, three other studies reported risk estimates
of wheezing in relation to workplace dampness or mold exposures. A study
from the United States reported an OR of 2.8 for usual wheezing and 1.9
for occasional wheezing in association with mold exposure in problem
office buildings in Florida (22). A study of Taiwanese daycare
centers found an increased risk of wheezing in relation to stuffy odor
(OR, 1.38), visible mold (OR, 1.39), and water damage (OR, 1.32) (21).
Another Taiwanese study of office workers found similar ORs for chest
tightness and chest pain (10).
Two Dutch studies
assessed the risk of asthma related to residential dampness stratified
by sex. In one of them (18) the risk was similar in men (OR,
1.29) and women (OR, 1.25), whereas the other (13) found, in
agreement with our study, a greater risk in women (OR, 4.16) than in men
(OR, 1.15). The Swedish study (20) found an essentially similar
risk of asthma related to visible mold growth among men (2.7) and women
(2.0). Modification by age, genetic predisposition, or smoking status
has not been previously studied.
The present results
provide new evidence of the relation between workplace exposure to
indoor molds and development of asthma in adulthood. Our findings
suggest that indoor mold problems constitute an important occupational
health hazard.




